Brief communication: Long-term treatment outcomes of transitioning to dolutegravir-based ART from efavirenz in HIV study participants in Mbeya, Tanzania
- PMID: 39716209
- PMCID: PMC11667906
- DOI: 10.1186/s12981-024-00662-z
Brief communication: Long-term treatment outcomes of transitioning to dolutegravir-based ART from efavirenz in HIV study participants in Mbeya, Tanzania
Abstract
Background: The World Health Organization recommends dolutegravir-based antiretroviral therapy (ART) as the preferred first-line regimen for HIV treatment. This retrospective cohort study evaluated the long-term virologic outcomes and safety of transitioning from an efavirenz-based regimen (tenofovir, lamivudine, efavirenz [TLE]) to a dolutegravir-based regimen (tenofovir, lamivudine, dolutegravir [TLD]) among adult HIV participants in Mbeya, Tanzania.
Methods: Medical records of 250 adult HIV participants who transitioned from TLE to TLD at Mbeya Zonal Referral Hospital were reviewed from August 2022 to December 2022. The primary outcome was virologic failure, defined as HIV RNA > 1000 copies/mL. Secondary outcomes included viral suppression (< 50 copies/mL) and adverse drug reactions (ADRs). Using appropriate statistical tests, participant characteristics and outcomes were compared before and six months after transitioning.
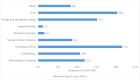
Results: At baseline on TLE, 88% had viral suppression, and 3.6% had virologic failure. Six months after transitioning to TLD, viral suppression was 87.2% and virologic failure increased to 6.8%. Overall, 79.6% experienced ADRs with TLD, predominantly neurological effects and weight gain. No significant associations were found between viral load changes and participant characteristics like age, sex or treatment duration.
Conclusions: Transitioning to dolutegravir maintained high rates of viral suppression comparable to efavirenz, albeit with a slight increase in virologic failure. Dolutegravir was well-tolerated overall despite a high ADR rate. Findings support the ongoing scale-up of dolutegravir in Tanzania and other resource-limited settings while highlighting the need for continued viral load monitoring and pharmacovigilance.
Keywords: Dolutagravir-based antiretroviral therapy; Efavirenz-based antiretroviral therapy; HIV-infected study participants; Retrospective evaluation; Tanzania; Treatment outcomes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This study received approval from the University of Dar es Salaam (UDSM), Mbeya College of Health and Allied Sciences (MCHAS) Research Ethical Clearance Sub-Committee (Ref No.: 2018-04-13276). The confidentiality and privacy of participants’ information were protected during the record review. Identifying details such as names were excluded from the records to maintain anonymity. Since this retrospective study reviewed existing medical records, individual consent was unnecessary. However, MZRH administration granted a waiver of informed consent while ensuring appropriate protections were in place to safeguard participants’ rights and privacy. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www.who.int/publications-detail-redirect/9789240031593. Accessed 6 Aug 2023. - PubMed
-
- World Health Organization. United Republic of Tanzania: HIV Country Profile: 2017. World Health Organisation; 2020.
-
- Ministry of Health. Community Development, gender, Elderly and Children (MoHCDGEC). National Guideline for the management of HIV and AIDS. National AIDS Control Programme; 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials