Prostanoid signaling in retinal cells elicits inflammatory responses relevant to early-stage diabetic retinopathy
- PMID: 39716241
- PMCID: PMC11667846
- DOI: 10.1186/s12974-024-03319-w
Prostanoid signaling in retinal cells elicits inflammatory responses relevant to early-stage diabetic retinopathy
Abstract
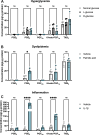
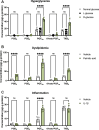
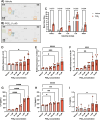
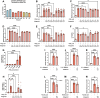
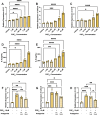
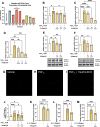
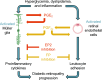
Inflammation is a critical driver of the early stages of diabetic retinopathy (DR) and offers an opportunity for therapeutic intervention before irreversible damage and vision loss associated with later stages of DR ensue. Nonsteroidal anti-inflammatory drugs (NSAIDs) have shown mixed efficacy in slowing early DR progression, notably including severe adverse side effects likely due to their nonselective inhibition of all downstream signaling intermediates. In this study, we investigated the role of prostanoids, the downstream signaling lipids whose production is inhibited by NSAIDs, in promoting inflammation relevant to early-stage DR in two human retinal cell types: Müller glia and retinal microvascular endothelial cells. When cultured in multiple conditions modeling distinct aspects of systemic diabetes, Müller glia significantly increased production of prostaglandin E2 (PGE2), whereas retinal endothelial cells significantly increased production of prostaglandin F2α (PGF2α). Müller glia stimulated with PGE2 or PGF2α increased proinflammatory cytokine levels dose-dependently. These effects were blocked by selective antagonists to the EP2 receptor of PGE2 or the FP receptor of PGF2α, respectively. In contrast, only PGF2α stimulated adhesion molecule expression in retinal endothelial cells and leukocyte adhesion to cultured endothelial monolayers, effects that were fully prevented by FP receptor antagonist treatment. Together these results identify PGE2-EP2 and PGF2α-FP signaling as novel, selective targets for future studies and therapeutic development to mitigate or prevent retinal inflammation characteristic of early-stage DR.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. All human materials were obtained from the National Disease Research Interchange or commercial sources and de-identified. Patient identity cannot be ascertained by the investigators. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy.Exp Eye Res. 2014 Aug;125:79-88. doi: 10.1016/j.exer.2014.05.012. Epub 2014 Jun 6. Exp Eye Res. 2014. PMID: 24910902 Free PMC article.
-
The peroxisome proliferator-activated receptor-β/δ antagonist GSK0660 mitigates retinal cell inflammation and leukostasis.Exp Eye Res. 2020 Jan;190:107885. doi: 10.1016/j.exer.2019.107885. Epub 2019 Nov 20. Exp Eye Res. 2020. PMID: 31758977 Free PMC article.
-
Nuclear factor of activated T-cells (NFAT) regulation of IL-1β-induced retinal vascular inflammation.Biochim Biophys Acta Mol Basis Dis. 2021 Dec 1;1867(12):166238. doi: 10.1016/j.bbadis.2021.166238. Epub 2021 Jul 31. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34343639 Free PMC article.
-
The role of Müller cell glucocorticoid signaling in diabetic retinopathy.Graefes Arch Clin Exp Ophthalmol. 2020 Feb;258(2):221-230. doi: 10.1007/s00417-019-04521-w. Epub 2019 Nov 16. Graefes Arch Clin Exp Ophthalmol. 2020. PMID: 31734719 Review.
-
Endothelial Dysfunction in Diabetic Retinopathy.Front Endocrinol (Lausanne). 2020 Sep 4;11:591. doi: 10.3389/fendo.2020.00591. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33013692 Free PMC article. Review.
Cited by
-
Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks.Pharmaceutics. 2025 Jun 17;17(6):791. doi: 10.3390/pharmaceutics17060791. Pharmaceutics. 2025. PMID: 40574103 Free PMC article.
References
-
- Kempen JH, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. - PubMed
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012. 10.1056/NEJMra1005073. - PubMed
-
- Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Prim. 2016;2:1–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

