Hypoglycemic agents and bone health; an umbrella systematic review of the clinical trials' meta-analysis studies
- PMID: 39716250
- PMCID: PMC11665059
- DOI: 10.1186/s13098-024-01518-2
Hypoglycemic agents and bone health; an umbrella systematic review of the clinical trials' meta-analysis studies
Abstract
Background: No clear consensus exists regarding the safest anti-diabetic drugs with the least adverse events on bone health. This umbrella systematic review therefore aims to assess the published meta-analysis studies of randomized controlled trials (RCTs) conducted in this field.
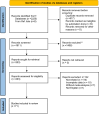
Methods: All relevant meta-analysis studies of RCTs assessing the effects of anti-diabetic agents on bone health in patients with diabetes mellitus (DM) were collected in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). English articles published until 15 March 2023 were collected through the search of Cochrane Library, Scopus, ISI Web of Sciences, PubMed, and Embase using the terms "Diabetes mellitus", "anti-diabetic drugs", "Bone biomarker", "Bone fracture, "Bone mineral density" and their equivalents. The methodological and evidence quality assessments were performed for all included studies.
Results: From among 2220 potentially eligible studies, 71 meta-analyses on diabetic patients were included. Sodium-glucose cotransporter-2 inhibitors (SGLT-is) showed no or equivalent effect on the risk of fracture. Dipeptidyl peptidase-4 inhibitors (DPP-4is) and Glucagon-like peptide-1 receptor agonists (GLP-1Ras) were reported to have controversial effects on bone fracture, with some RCTs pointing out the bone protective effects of certain members of these two medication classes. Thiazolidinediones (TZDs) were linked with increased fracture risk as well as higher concentrations of C-terminal telopeptide of type I collagen (CTx), a bone resorption marker.
Conclusion: The present systematic umbrella review observed varied results on the association between the use of anti-diabetic drugs and DM-related fracture risk. The clinical efficacy of various anti-diabetic drugs, therefore, should be weighed against their risks and benefits in each patient.
Keywords: Anti-diabetic agents; Bone biomarkers; Bone fracture; Bone mineral density; Diabetes mellitus.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was granted by Research Ethics Committees of Endocrine & Metabolism Research Institute- Tehran University of Medical Sciences (IR.TUMS.EMRI.REC.1401.155). Competing interests: The authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources