Accumulated BCAAs and BCKAs contribute to the HFD-induced deterioration of Alzheimer's disease via a dysfunctional TREM2-related reduction in microglial β-amyloid clearance
- PMID: 39716292
- PMCID: PMC11667870
- DOI: 10.1186/s12974-024-03314-1
Accumulated BCAAs and BCKAs contribute to the HFD-induced deterioration of Alzheimer's disease via a dysfunctional TREM2-related reduction in microglial β-amyloid clearance
Abstract
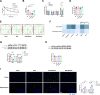
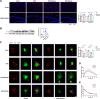
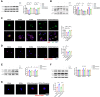
A high-fat diet (HFD) induces obesity and insulin resistance, which may exacerbate amyloid-β peptide (Aβ) pathology during Alzheimer's disease (AD) progression. Branched-chain amino acids (BCAAs) accumulate in obese or insulin-resistant patients and animal models. However, roles of accumulated BCAAs and their metabolites, branched-chain keto acids (BCKAs), in the HFD-induced deterioration of AD and the underlying mechanisms remains largely unclear. In this study, APPswe/PSEN1dE9 (APP/PS1) transgenic mice were fed a HFD for 6 months, and the BCAAs content of the HFD was adjusted to 200% or 50% to determine the effects of BCAAs. The HFD-fed APP/PS1 mice accumulated BCAAs and BCKAs in the serum and cortex, which was accompanied by more severe cognitive deficits and AD-related pathology. The additional or restricted intake of BCAAs aggravated or reversed these phenomena. Importantly, BCAAs and BCKAs repressed microglial phagocytosis of Aβ in vivo and in BV2 cells, which might be relevant for triggering receptor expressed on myeloid cells 2 (TREM2) dysfunction and autophagy deficiency. We found that BCAAs and BCKAs could bind to TREM2 in silico, in pure protein solutions and in the cellular environment. These molecules competed with Aβ for binding to TREM2 so that the response of TREM2 to Aβ was impaired. Moreover, BCAAs and BCKAs decreased TREM2 recycling in an mTOR-independent manner, which might also lead to TREM2 dysfunction. Our findings suggest that accumulated BCAAs and BCKAs contribute to the HFD-induced acceleration of AD progression through hypofunctional TREM2-mediated disturbances in Aβ clearance in microglia. Lowering BCAAs and BCKAs levels may become a potential dietary intervention for AD.
Keywords: Alzheimer’s disease; Amyloid-β; BCAAs; BCKAs; HFD; Insulin resistance; Microglia; TREM2.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures of animals were approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IACUC number: 2946), which complied with animal biomedical research principles formulated by the China Animal Care Committee and the Council of the International Medical Organization. Competing interests: The authors declare no competing interests.
Figures



References
-
- De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–72. - PubMed
-
- Guo Y, Wang Q, Chen S, Xu C. Functions of amyloid precursor protein in metabolic diseases. Metabolism. 2021;115: 154454. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

