Gegen-Sangshen oral liquid and its active fractions mitigate alcoholic liver disease in mice through repairing intestinal epithelial injury and regulating gut microbiota
- PMID: 39716295
- PMCID: PMC11667864
- DOI: 10.1186/s13020-024-01049-y
Gegen-Sangshen oral liquid and its active fractions mitigate alcoholic liver disease in mice through repairing intestinal epithelial injury and regulating gut microbiota
Abstract
Background: Liuweizhiji Gegen-Sangshen oral liquid (LGS), as a Chinese medicinal preparation, is developed from a Traditional Chinese medicinal formula consisting of six Chinese medicinal herbs, including Puerariae lobatae radix, Hoveniae semen, Imperatae rhizoma, Crataegi fructus, Mori fructus and Canarli fructus, and has been extensively utilized in the prevention and treatment of alcoholic liver disease (ALD) clinically. Previous study has demonstrated that LGS dose-dependently mitigated ALD in rat models. However, whether and how the main characteristic constituents of LGS (the flavonoid and polysaccharide fractions, LGSF and LGSP) contribute to the anti-ALD effect remains unclear. This study aimed to assess the anti-ALD effect of LGS and its main fractions (LGSF and LGSP) in a murine model of ALD and to explore the underlying mechanisms.
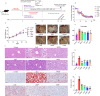
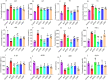
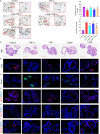
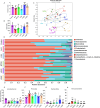
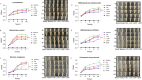
Methods: ALD mouse model was constructed using the chronic and binge ethanol feeding method. Biochemical determinations of AST, ALT, TC, TG, ADH, ALDH, HDL, LDL, IL-1β, IL-6, and TNF-α were performed using corresponding kits. Histopathological examination of liver and intestinal sections was conducted based on the H&E staining. Lipid accumulation in hepatocytes was evaluated by oil red O staining. Ethanol metabolism was assessed by determining the activity of ADH and ALDH enzymes. Intestinal barrier function was analyzed based on immunohistochemistry analysis of ZO-1 and occludin and immunofluorescence analysis of epithelial markers, Lgr5, Muc2, and Lyz1. Intestinal epithelial apoptosis was detected by TUNEL staining. Mouse fecal microbiota alterations were analyzed by 16S rRNA sequencing. An in vitro epithelial injury model was established by developing TNF-α-induced 3D-cultured intestinal organoids. In vitro culture of specific bacterial strains was performed.
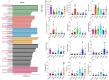
Results: The results showed that LGS and its flavonoid and polysaccharide fractions (LGSF and LGSP) significantly alleviated ALD in mice through attenuating hepatic injury and inflammation, improving liver steatosis and promoting ethanol metabolism. Notably, LGS, LGSP, and LGSF mitigated intestinal damage and maintained barrier function in ALD mice. The intestinal barrier protection function of LGS, LGSP, and LGSF was generally more obvious than that of the positive drug meltadosine. Further study demonstrated that LGS, LGSP, and LGSF promoted intestinal epithelial repair via promoting Lgr5+ stem cell mediated regeneration in TNF-α-induced intestinal organoids. LGS and LGSF, other than LGSP, had a better effect on repair of epithelial injury in vitro. Moreover, LGS, LGSP, and LGSF remarkably alleviated gut dysbiosis in ALD mice via at least partially recovery of alcohol-induced microbial changes and induction of specific bacterial groups. In vitro culture of bacterial strains indicated that LGS, LGSP, and LGSF had a specific impact on bacterial growth. LGS and LGSP, but not the LGSF, significantly promoted the growth of Lactobacillus. Similarly, LGS and LGSP significantly increased the proliferation of Bacteroides sartorii, and LGSF had a minimal effect. LGS, LGSP and LGSF all promoted the growth of Bacillus coagulans, Bifidobacterium adolescentis, and Bifidobacterium bifidum. LGS and LGSP promoted the growth of Dubosiella newyorkensis, but the LGSF had no effect.
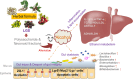
Conclusions: LGS exerts its anti-ALD effect in mice through regulating gut-liver axis, and its flavonoid and polysaccharide fractions, LGSF and LGSP, are responsible for its protective effect.
Keywords: Alcoholic liver disease; Gegen-Sangshen oral liquid; Gut microbiota; Gut-liver axis; Intestinal organoid.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were approved by the Committee on the Ethics of Animal Experiments of Southwest Medical University (Approval No. 20221026–022). Consent for publication: Not applicable. Competing interests: The authors report no competing interests in this work.
Figures

Similar articles
-
Liuweizhiji Gegen-Sangshen beverage protects against alcoholic liver disease in mice through the gut microbiota mediated SCFAs/GPR43/GLP-1 pathway.Front Nutr. 2024 Dec 13;11:1495695. doi: 10.3389/fnut.2024.1495695. eCollection 2024. Front Nutr. 2024. PMID: 39734674 Free PMC article.
-
Fingerprint profiling for quality evaluation and the related biological activity analysis of polysaccharides from Liuweizhiji Gegen-Sangshen beverage.Front Nutr. 2024 Jul 8;11:1431518. doi: 10.3389/fnut.2024.1431518. eCollection 2024. Front Nutr. 2024. PMID: 39040925 Free PMC article.
-
Lacticaseibacillus paracasei 36 Mitigates Alcoholic-Associated Liver Disease Through Modulation of Microbiota and AMPK Signaling.Nutrients. 2025 Jul 17;17(14):2340. doi: 10.3390/nu17142340. Nutrients. 2025. PMID: 40732967 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
The Role of Gut Bacteria and Fungi in Alcohol-Associated Liver Disease.Front Med (Lausanne). 2022 Mar 3;9:840752. doi: 10.3389/fmed.2022.840752. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35308525 Free PMC article. Review.
References
-
- Osna NA, Tikhanovich I, Ortega-Ribera M, Mueller S, Zheng C, Mueller J, Li S, Sakane S, Weber RCG, Kim HY, Lee W, Ganguly S, Kimura Y, Liu X, Dhar D, Diggle K, Brenner DA, Kisseleva T, Attal N, McKillop IH, Chokshi S, Mahato R, Rasineni K, Szabo G, Kharbanda KK. Alcohol-associated liver disease outcomes: critical mechanisms of liver injury progression. Biomolecules. 2024;14:404. - PMC - PubMed
-
- Wang W, Liu M, Fu X, Qi M, Zhu F, Fan F, Wang Y, Zhang K, Chu S. Hydroxysafflor yellow A ameliorates alcohol-induced liver injury through PI3K/Akt and STAT3/NF-κB signaling pathways. Phytomedicine. 2024;132: 155814. - PubMed
-
- Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

