Treatment initiation and completion among head and neck squamous cell carcinoma patients in Tanzania
- PMID: 39716326
- PMCID: PMC11668109
- DOI: 10.1186/s13104-024-07045-7
Treatment initiation and completion among head and neck squamous cell carcinoma patients in Tanzania
Abstract
Objective: Few studies characterizing clinical outcomes of head and neck cancer (HNC) patients in sub-Saharan Africa report the proportion of patients who initiate and complete treatment, information integral to contextualizing survival outcomes. This retrospective cohort study describes HNC patients who presented to Muhimbili National Hospital and Ocean Road Cancer Institute in 2018, the highest-volume oncology tertiary referral centers in Tanzania. Logistic regression was applied to assess predictors of treatment initiation and completion.
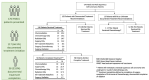
Results: Among the 176 head and neck squamous cell carcinoma (HNSCC) patients, 34% (59) had no treatment documented, 34%(59) had documentation of treatment initiation but not completion, and 33%(58) had documentation of treatment completion based on the modalities started. Univariate logistic regression showed that late-stage disease was associated with increased odds of initiating treatment (OR 8.24, 95% CI 2.05-33.11, p = 0.003) and trends toward completing treatment (OR 7.41, 95% CI 0.90-60.99, p = 0.063). At last visit, 36.9%(65) were alive with a median follow up of 5.6 months (IQR 1.64-12.5 months). A large proportion of HNC patients who presented to MNH and ORCI did not initiate or complete treatment. These metrics are critical to contextualize care outcomes of HNC patients in resource-constrained health systems and develop interventions.
Keywords: Africa; Head and neck cancer; Tanzania; Treatment incompletion.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the institutional review boards of the University of California San Francisco (IRB 18-26808), Muhimbili University of Health and Allied Sciences (REC-11-2019-064), and by Tanzania’s National Institute of Medical Research (NIMR/HQ/R.8a/vol.IX/3394). Informed consent was waived by institutional review boards of the University of California San Francisco (IRB 18-26808), Muhimbili University of Health and Allied Sciences (REC-11-2019-064), and by Tanzania’s National Institute of Medical Research (NIMR/HQ/R.8a/vol.IX/3394). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Ouni SBN, Khaireddine N, Abdelkefi M, Bouaouina N. Nasopharyngeal carcinoma in Tunisian centre: about 712 cases le cancer Du cavum dans le centre Tunisien: a propos de 712 cas. Eur J Pharm Med Res. 2018;5:523–9.
-
- Olusanya AA, Adisa AO, Aladelusi TO, Lawal OA, Adeyemi BF, Gbolahan OO, et al. Orofacial cancers: Pattern and Management in Ibadan, Nigeria. Med J Zambia. 2018;45:179–88.
-
- Roos ME, Sherriff A, Seedat R. Laryngeal squamous cell carcinoma at a South African referral hospital. Egypt J Ear Nose Throat Allied Sci. 2019;20(2):53–9.