Triphenyl-Modified Mixed-Mode Stationary Phases With and Without Embedded Ion-Exchange Sites for High-Performance Liquid Chromatography
- PMID: 39716842
- PMCID: PMC11667146
- DOI: 10.1002/jssc.70058
Triphenyl-Modified Mixed-Mode Stationary Phases With and Without Embedded Ion-Exchange Sites for High-Performance Liquid Chromatography
Abstract
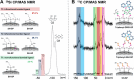
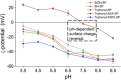
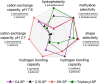
The present work reports on the preparation, characterization, and evaluation of a set of novel triphenyl-modified silica-based stationary phases without and with embedded ion-exchange sites for mixed-mode liquid chromatography. The three synthesized triphenyl phases differed in additionally incorporated ion-exchange sites. In one embodiment, allyltriphenylsilane was bonded to thiol-modified silica by thiol-ene click reaction, leading to particles with no ion-exchange sites. A second stationary phase was obtained by thiol-yne click reaction of thiol silica with 2-propinyl-triphenylphosphonium bromide, yielding a strong anion-exchanger (SAX). A third stationary phase was obtained from this SAX phase by the oxidation of residual thiols to sulfonic acid moieties, leading to a zwitterionic surface. All synthesized materials were subjected to elemental analysis, 13C and 29Si solid-state cross-polarization/magic angle spinning nuclear magnetic resonance (CP/MAS NMR) spectroscopy analysis, and pH-dependent ζ-potential determinations via electrophoretic light scattering. The prepared stationary phases were chromatographically evaluated under classical reversed-phase, ion-exchange, and hydrophilic interaction chromatography conditions and classified within a set of commercially available columns by principal component analysis of retention factors. Finally, the obtained stationary phases were applied for biomolecule separations (e.g., teicoplanin and siRNA patisiran). These LC tests proved the orthogonality of the three prepared stationary phases and indicated possible fields of application.
Keywords: mixed‐mode chromatography; oligonucleotide; silatrane; stationary phase; thiol‐yne/ene click reaction.
© 2024 The Author(s). Journal of Separation Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Sykora D., Rezanka P., Záruba K., and Král V., “Recent Advances in Mixed‐Mode Chromatographic Stationary Phases,” Journal of Separation Science 42 (2019): 89–129. - PubMed
-
- Wang L. J., Wei W. L., Xia Z. N., Jie X., and Xia Z. Z. L., “Recent Advances in Materials for Stationary Phases of Mixed‐Mode High‐Performance Liquid Chromatography,” TRAC‐Trends in Analytical Chemistry 80 (2016): 495–506.
-
- Zhang L., Dai Q., Qiao X. Q., Yu C. Y., Qin X. Y., and Yan H. Y., “Mixed‐Mode Chromatographic Stationary Phases: Recent Advancements and Its Applications for High‐Performance Liquid Chromatography,” TRAC‐Trends in Analytical Chemistry 82 (2016): 143–163.
-
- Zhang K. and Liu X. D., “Mixed‐Mode Chromatography in Pharmaceutical and Biopharmaceutical Applications,” Journal of Pharmaceutical and Biomedica 128 (2016): 73–88. - PubMed
-
- Laemmerhofer M., Richter M., Wu J. Y., Nogueira R., Bicker W., and Lindner W., “Mixed‐Mode Ion‐Exchangers and Their Comparative Chromatographic Characterization in Reversed‐Phase and Hydrophilic Interaction Chromatography Elution Modes,” Journal of Separation Science 31 (2008): 2572–2588. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

