A phase 1/2 safety and efficacy study of TAK-754 gene therapy: The challenge of achieving durable factor VIII expression in haemophilia A clinical trials
- PMID: 39716875
- PMCID: PMC11780198
- DOI: 10.1111/hae.15121
A phase 1/2 safety and efficacy study of TAK-754 gene therapy: The challenge of achieving durable factor VIII expression in haemophilia A clinical trials
Abstract
Introduction: Haemophilia A is an X-linked bleeding disorder resulting from a deficiency of factor VIII (FVIII). To date, multiple gene therapies have entered clinical trials with the goal of providing durable haemostatic protection from a single dose. TAK 754 (BAX 888) is an investigational AAV8-based gene therapy containing a FVIII transgene. Reduction in CpG motifs was performed to reduce immunogenicity based on prior observations. Here, we describe the results of the first two cohorts treated with TAK 754.
Aim: To report clinical and translational results of the TAK-754 phase 1/2 AAV gene therapy study for the treatment of haemophilia A.
Methods: A phase 1/2 single arm open-label dose escalation study of TAK-754 was performed in participants with severe haemophilia A (NCT03370172). Participants were monitored for safety events, endogenous FVIII activity and bleeding rates. Glucocorticoids were implemented to preserve transgene expression. A transcriptomics analysis was performed to evaluate immunogenicity along with additional post-hoc analyses.
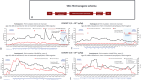
Results: Four participants were dosed in two cohorts. Infusion of TAK 754 was well-tolerated. All participants developed mild transient transaminase elevation and subsequent loss of FVIII expression within the first 12 months of treatment despite use of glucocorticoids. Transcriptomic analysis did not demonstrate significant changes in immunogenicity signals in peripheral blood. One serious adverse event of hypophosphatemia occurred in the second cohort without obvious risk factors.
Conclusions: Sustained FVIII expression remains a challenge in haemophilia A AAV gene therapy trials. Mechanisms of transgene expression loss require further study as clinical studies enter long term follow-up periods.
Keywords: adeno‐associated virus; gene therapy; haemophilia; hypophosphatemia; phase 1–2.
© 2024 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
J.C., M.A.R., K.R., M.W., J.K., D.D., M.G., M.P.W., Q.W. are employees and shareholders of Takeda Pharmaceuticals, Ltd. C.M. is an employee of IQIVIA. M.T.A.R. has received honoraria for speaking engagements or consulting or research funds from Takeda, Bayer, CSL Behring, Grifols, Novo Nordisk, Sobi, Octapharma, Amgen, Novartis, and Pfizer. F.J.L.J. has participated as speaker, in advisory boards and sponsored symposia, with Amgen, Bayer, CSL Behring, Leo Pharma, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, and Takeda. S.S. reports research support from CorWave, Roche‐Chugai, Stago; is a trial investigator for Biomarin, Bioverativ, CSL Behring, LFB, Sanofi, Shire/Takeda, Siemens, Healthiners, Sanofi and Sobi; has served as a speaker or advisor for CSL Behring, LFB, Novo‐Nordisk, Roche, Siemens, SobiHonoraria, Biomarin,Pfizer, Sanofi, Stago, and Takeda. AR, MEMC report no conflict of interest.
Figures

References
-
- von Drygalski A, Chowdary P, Kulkarni R, et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med. 2023;388(4):310‐318. - PubMed
-
- Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811‐822. - PubMed
-
- Berntorp E, Hermans C, Solms A, Poulsen L, Mancuso ME. Optimising prophylaxis in haemophilia A: the ups and downs of treatment. Blood Rev. 2021;50:100852. - PubMed
-
- Mahlangu J, Kaczmarek R, von Drygalski A, et al. Two‐year outcomes of valoctocogene roxaparvovec therapy for hemophilia A. N Engl J Med. 2023;388(8):694‐705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

