From bugs to brain: unravelling the GABA signalling networks in the brain-gut-microbiome axis
- PMID: 39716883
- PMCID: PMC12074267
- DOI: 10.1093/brain/awae413
From bugs to brain: unravelling the GABA signalling networks in the brain-gut-microbiome axis
Abstract
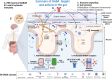
Convergent data across species paint a compelling picture of the critical role of the gut and its resident microbiota in several brain functions and disorders. The chemicals mediating communication along these sophisticated highways of the brain-gut-microbiome (BGM) axis include both microbiota metabolites and classical neurotransmitters. Amongst the latter, GABA is fundamental to brain function, mediating most neuronal inhibition. Until recently, GABA's role and specific molecular targets in the periphery within the BGM axis had received limited attention. Yet, GABA is produced by neuronal and non-neuronal elements of the BGM, and recently, GABA-modulating bacteria have been identified as key players in GABAergic gut systems, indicating that GABA-mediated signalling is likely to transcend physiological boundaries and species. We review the available evidence to better understand how GABA facilitates the integration of molecularly and functionally disparate systems to bring about overall homeostasis and how GABA perturbations within the BGM axis can give rise to multi-system medical disorders, thereby magnifying the disease burden and the challenges for patient care. Analysis of transcriptomic databases revealed significant overlaps between GABAAR subunits expressed in the human brain and gut. However, in the gut, there are notable expression profiles for a select number of subunits that have received limited attention to date but could be functionally relevant for BGM axis homeostasis. GABAergic signalling, via different receptor subtypes, directly regulates BGM homeostasis by modulating the excitability of neurons within brain centres responsible for gastrointestinal (GI) function in a sex-dependent manner, potentially revealing mechanisms underlying the greater prevalence of GI disturbances in females. Apart from such top-down regulation of the BGM axis, a diverse group of cell types, including enteric neurons, glia, enteroendocrine cells, immune cells and bacteria, integrate peripheral GABA signals to influence brain functions and potentially contribute to brain disorders. We propose several priorities for this field, including the exploitation of available technologies to functionally dissect components of these GABA pathways within the BGM, with a focus on GI and brain-behaviour-disease. Furthermore, in silico ligand-receptor docking analyses using relevant bacterial metabolomic datasets, coupled with advances in knowledge of GABAAR 3D structures, could uncover new ligands with novel therapeutic potential. Finally, targeted design of dietary interventions is imperative to advancing their therapeutic potential to support GABA homeostasis across the BGM axis.
Keywords: GABA receptors; enteric nervous system; inflammation; neurosteroids; pre/probiotics; psychopathology.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare that this manuscript was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. J.D.S. is in receipt of research funding from GABA Labs. D.B. works as a senior scientific consultant for GABA Labs and as a Visiting Researcher at the University of Portsmouth. All other authors report no competing interests.
Figures




References
-
- Cryan JF, O’Riordan KJ, Cowan CSM, et al. . The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. - PubMed
-
- Morais LH, Schreiber HL 4th, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–255. - PubMed
-
- Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

