Human umbilical cord mesenchymal stem cells enhance liver regeneration and decrease collagen content in fibrosis mice after partial hepatectomy by activating Wnt/β-catenin signaling
- PMID: 39716885
- PMCID: PMC12040747
- DOI: 10.3724/abbs.2024207
Human umbilical cord mesenchymal stem cells enhance liver regeneration and decrease collagen content in fibrosis mice after partial hepatectomy by activating Wnt/β-catenin signaling
Abstract
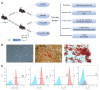
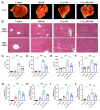
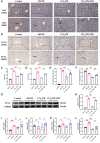
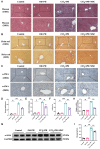
Liver fibrosis is a critical stage in the progression of various chronic liver diseases to cirrhosis and liver cancer. Early inhibition of liver fibrosis is crucial for the treatment of liver disease. Hepatectomy, a common treatment for liver-related diseases, promotes liver regeneration. However, in the context of liver fibrosis, liver regeneration is hindered. Many studies have shown that mesenchymal stem cells (MSCs) can promote liver regeneration after partial hepatectomy (PH). However, there are few reports on the impact of MSC therapy on liver regeneration post-PH in the context of hepatic fibrosis. The objective of this study is to examine the impact of MSCs on liver regeneration following PH in the fibrotic liver and uncover the related molecular mechanisms. This study reveals that MSC therapy significantly enhances liver function and mitigates liver inflammation after PH in the context of hepatic fibrosis. MSCs also significantly promote liver regeneration and alleviate liver fibrosis. In addition, this study identifies the role of MSCs in promoting liver regeneration and alleviating liver fibrosis via the activation of Wnt/β-catenin signaling. The combination of MSCs with hepatectomy may offer a novel approach for the treatment of liver fibrotic diseases.
Keywords: liver fibrosis; liver regeneration; mesenchymal stem cells; partial hepatectomy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Ohtani N, Kawada N. Role of the gut–liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatol Commun. . 2019;3:456–470. doi: 10.1002/hep4.1331. - DOI - PMC - PubMed
-
- Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, Yoshiji H, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol. . 2019;54:367–376. doi: 10.1007/s00535-018-1532-5. - DOI - PMC - PubMed
-
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) . 2008;233:109–122. doi: 10.3181/0707-MR-190. - DOI - PubMed
-
- Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. . 2015;39:S60–S63. doi: 10.1016/j.clinre.2015.06.015. - DOI - PMC - PubMed
-
- Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. . 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

