TTK Inhibition Alleviates Postinjury Neointimal Formation and Atherosclerosis
- PMID: 39716891
- PMCID: PMC11809377
- DOI: 10.1002/advs.202409250
TTK Inhibition Alleviates Postinjury Neointimal Formation and Atherosclerosis
Abstract
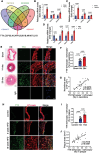
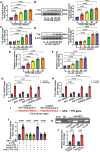
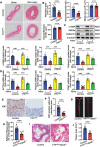
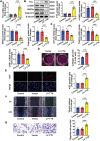
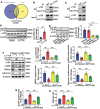
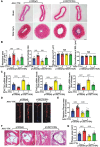
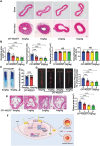
Atherosclerosis and its associated cardio-cerebrovascular complications remain the leading causes of mortality worldwide. Current lipid-lowering therapies reduce only approximately one-third of the cardiovascular risk. Furthermore, vascular restenosis and thrombotic events following surgical interventions for severe vascular stenosis significantly contribute to treatment failure. This highlights the urgent need for novel therapeutic targets to manage atherosclerosis and prevent restenosis and thrombosis after vascular injury. This study identifies TTK protein kinase (TTK) as a key regulator of vascular smooth muscle cell (VSMC) phenotypic switching in the context of postinjury neointimal formation and atherosclerosis. Mechanistically, TTK upregulation in VSMCs phosphorylates p120-catenin, leading to β-catenin nuclear accumulation and dissociation of the myocardin (MYOCD)/serum response factor (SRF) complex. Deletion of TTK specifically in VSMCs reduces postinjury neointimal formation in vascular injury models and attenuates atherosclerotic lesions in ApoE-/- mice. Notably, oral administration of the TTK inhibitor CFI-402257 mitigated neointimal formation without impairing reendothelialization and reduced atherosclerotic lesions in ApoE-/- mice without altering lipid levels. These findings suggest that targeting TTK, through inhibitors or alternative strategies, represents a promising approach to simultaneously prevent postinjury restenosis and treat atherosclerosis.
Keywords: TTK; atherosclerosis; p120‐catenin; restenosis; vascular smooth muscle cells.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Libby P., Nature 2021, 592, 524. - PubMed
-
- Crea F., Eur. Heart J. 2023, 44, 1287. - PubMed
-
- Borovac J. A., D'Amario D., Vergallo R., Porto I., Bisignani A., Galli M., Annibali G., Montone R. A., Leone A. M., Niccoli G., Crea F., Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 105. - PubMed
-
- Ma Z., Mao C., Chen X., Yang S., Qiu Z., Yu B., Jia Y., Wu C., Wang Y., Wang Y., Gu R., Yu F., Yin Y., Wang X., Xu Q., Liu C., Liao Y., Zheng J., Fu Y., Kong W., Circulation 2023, 147, 728. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
