Senescent Fibroblasts Drive FAP/OLN Imbalance Through mTOR Signaling to Exacerbate Inflammation and Bone Resorption in Periodontitis
- PMID: 39716898
- PMCID: PMC11831441
- DOI: 10.1002/advs.202409398
Senescent Fibroblasts Drive FAP/OLN Imbalance Through mTOR Signaling to Exacerbate Inflammation and Bone Resorption in Periodontitis
Abstract
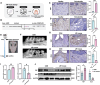
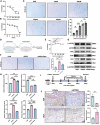
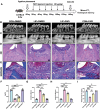
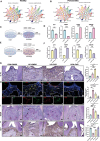
Fibroblast activation protein (FAP), predominantly expressed in activated fibroblasts, plays a key role in inflammatory bone diseases, but its role in periodontitis remains unclear. Accordingly, this study identified a positive association between FAP levels and periodontitis susceptibility using Mendelian randomization analysis. Human and mouse periodontitis tissues show elevated FAP and reduced osteolectin (OLN), an endogenous FAP inhibitor, indicating a FAP/OLN imbalance. Single-cell RNA sequencing revealed gingival fibroblasts (GFs) as the primary FAP and OLN source, with periodontitis-associated GFs showing increased reactive oxygen species, cellular senescence, and mTOR pathway activation. Rapamycin treatment restored the FAP/OLN balance in GFs. Recombinant FAP increased pro-inflammatory cytokine secretion and osteoclast differentiation in macrophages, exacerbating periodontal damage, whereas FAP inhibition reduced macrophage inflammation, collagen degradation, and bone resorption in experimental periodontitis. Therefore, senescent fibroblasts drive the FAP/OLN imbalance through mTOR activation, contributing to periodontitis progression. Consequently, targeting FAP may offer a promising therapeutic strategy for periodontitis.
Keywords: cellular senescence; fibroblast activation protein; macrophage; osteolectin; periodontitis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kinane D. F., Stathopoulou P. G., Papapanou P. N., Nat. Rev. Dis. Primers 2017, 3, 17038. - PubMed
-
- Nibali L., Farias B. C., Vajgel A., Tu Y. K., Donos N., J. Dent. Res. 2013, 92, 868. - PubMed
-
- Caffesse R. G., Echeverría J. J., Periodontol 2000 2019, 79, 7. - PubMed
-
- Kornman K. S., J Periodontol 2008, 79, 1560. - PubMed
-
- Sanz M., Herrera D., Kebschull M., Chapple I., Jepsen S., Berglundh T., Sculean A., Tonetti M. S., E. W. P. and M. Consultants , J. Clin. Periodontol. 2020, 47, 4. - PubMed
MeSH terms
Substances
Grants and funding
- S07924040B/Animal Research Ethics Committee of Wuhan University School of Stomatology and Hospital, China
- WDKQ2024B01/Ethics Committee of School & Hospital China Hospital of Stomatology Wuhan University
- 32370816/National Natural Science Foundation of China
- 82071095/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous
