Triple-Combination Therapy with a Multifunctional Yolk-Shell Nanozyme Au@CeO2 Loaded with Dimethyl Fumarate for Periodontitis
- PMID: 39716921
- PMCID: PMC11831482
- DOI: 10.1002/advs.202413891
Triple-Combination Therapy with a Multifunctional Yolk-Shell Nanozyme Au@CeO2 Loaded with Dimethyl Fumarate for Periodontitis
Abstract
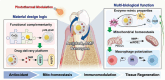
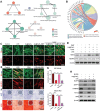
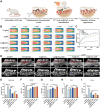
Periodontitis, a chronic inflammatory disease, is the leading cause of tooth loss in adults and is one of the most prevalent and complex oral conditions. Oxidative stress induced by the excessive generation of reactive oxygen species (ROS) leads to periodontitis, which is closely associated with pathological processes, including mitochondrial dysfunction of periodontal cells and local immune dysregulation. However, current treatment modalities that target single pathological processes have limited long-term therapeutic effects. Herein, a multifunctional Yolk-Shell nanozyme, Au@CeO2-dimethyl fumarate (DMF), which comprehensively addresses the oxidative stress-induced pathophysiological processes of periodontitis through antioxidant activity, mitochondrial maintenance, and immune modulation mechanisms, is described. For material design logic, functionally complementary Au and CeO2 formed an excellent photothermally regulated high-efficiency nanozyme, which also provided an ideal drug carrier for DMF. As for the therapeutic logic, Au@CeO2-DMF restores mitochondrial dysfunction and immune dysregulation, which also contributes to endogenous ROS elimination, thereby achieving long-term stable therapeutic effects. In a rat model, local Au@CeO2-DMF photothermal therapy effectively alleviated ROS-induced tissue damage and restored periodontal homeostasis. Altogether, this study presents a novel antioxidant nanozyme for managing alveolar bone loss under prolonged oxidative stress and demonstrates the importance of comprehensive intervention in key pathological processes in periodontitis treatment design.
Keywords: dimethyl fumarate; nanozymes; periodontitis; photothermal therapy; reactive oxygen species.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Near-infrared light-responsive copper-cerium bimetallic oxide nanozyme with antibacterial and antioxidant abilities for periodontitis therapy.Colloids Surf B Biointerfaces. 2025 Aug;252:114685. doi: 10.1016/j.colsurfb.2025.114685. Epub 2025 Apr 9. Colloids Surf B Biointerfaces. 2025. PMID: 40233479
-
Gold core@CeO2 halfshell Janus nanocomposites catalyze targeted sulfate radical for periodontitis therapy.J Control Release. 2024 Jun;370:600-613. doi: 10.1016/j.jconrel.2024.05.016. Epub 2024 May 14. J Control Release. 2024. PMID: 38735394
-
Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting the ROS-NFκB pathway.Nanoscale. 2022 Feb 17;14(7):2628-2637. doi: 10.1039/d1nr06043k. Nanoscale. 2022. PMID: 35088792
-
Advances of Oxidative Stress Impact in Periodontitis: Biomarkers and Effective Targeting Options.Curr Med Chem. 2024;31(38):6187-6203. doi: 10.2174/0109298673297545240507091410. Curr Med Chem. 2024. PMID: 38726786 Review.
-
The role of mitochondrial dysfunction in periodontitis: From mechanisms to therapeutic strategy.J Periodontal Res. 2023 Oct;58(5):853-863. doi: 10.1111/jre.13152. Epub 2023 Jun 18. J Periodontal Res. 2023. PMID: 37332252 Review.
Cited by
-
The next generation of dental treatments: Leveraging smart-responsive nanomaterials for unique oral environments.Mater Today Bio. 2025 Jul 5;33:102057. doi: 10.1016/j.mtbio.2025.102057. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40809405 Free PMC article. Review.
-
Nanozymes Empower Periodontitis Treatment: New Strategies and Clinical Application Prospects.Biomater Res. 2025 May 20;29:0210. doi: 10.34133/bmr.0210. eCollection 2025. Biomater Res. 2025. PMID: 40395276 Free PMC article. Review.
-
From mitochondria to immune networks: new mesenchymal stem cell strategies to treat periodontitis.Stem Cell Res Ther. 2025 Aug 29;16(1):470. doi: 10.1186/s13287-025-04619-5. Stem Cell Res Ther. 2025. PMID: 40877977 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
