Metabolic Reprogramming of Macrophages by Biomimetic Melatonin-Loaded Liposomes Effectively Attenuates Acute Gouty Arthritis in a Mouse Model
- PMID: 39717013
- PMCID: PMC11831490
- DOI: 10.1002/advs.202410107
Metabolic Reprogramming of Macrophages by Biomimetic Melatonin-Loaded Liposomes Effectively Attenuates Acute Gouty Arthritis in a Mouse Model
Abstract
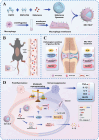
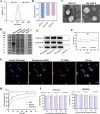
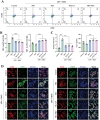
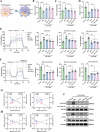
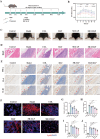
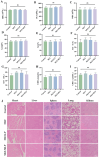
Gouty arthritis is characterized by an acute inflammatory response triggered by monosodium urate (MSU) crystals deposited in the joints and periarticular tissues. Current treatments bring little effects owing to serious side effects, necessitating the exploration of new and safer therapeutic options. Macrophages play a critical role in the initiation, progression, and resolution of acute gout, with the cellular profiles closely linked to their activation and polarization. This suggests that metabolic regulation can be of significance in managing gouty inflammation. In this study, it is demonstrated that melatonin, a natural hormone, modulates the metabolic remodeling of inflammatory macrophages by shifting their metabolism from glycolysis to oxidative phosphorylation, further altering functions of the pathogenic macrophage. To improve melatonin delivery to the inflamed sites, macrophage membrane-coated melatonin-loaded liposomes (MLT-MLP) are developed. Benefiting from the inflammation-homing characteristic of macrophage membrane, such engineered liposomes effectively target the inflamed site and demonstrate potent anti-inflammatory effects, achieving an enhanced amelioration of acute gouty arthritis. In conclusion, this study proposes a novel strategy aimed at metabolic reprogramming of macrophages to attenuate the pathological injuries in acute gout, providing a potential therapeutic strategy of gout-associated diseases, especially gouty arthritis.
Keywords: acute gout; biomimetic; macrophage; melatonin; metabolic reprogramming.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
