Sequential delivery of IL-10 and icariin using nanoparticle/hydrogel hybrid system for prompting bone defect repair
- PMID: 39717024
- PMCID: PMC11664418
- DOI: 10.1016/j.mtbio.2024.101374
Sequential delivery of IL-10 and icariin using nanoparticle/hydrogel hybrid system for prompting bone defect repair
Abstract
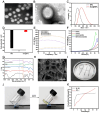
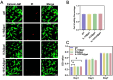
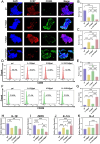
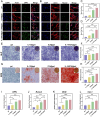
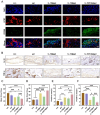
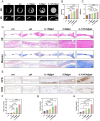
The treatment of large bone defects remains challenging due to the lack of spatiotemporal management of the immune microenvironment, inflammation response and bone remodeling. To address these issues, we designed and developed a nanoparticle/hydrogel hybrid system that can achieve the combined and sequential delivery of an anti-inflammatory factor (IL-10) and osteogenic drug (icariin, ICA). A photopolymerizable composite hydrogel was prepared by combining gelatin methacryloyl (GelMA) and heparin-based acrylated hyaluronic acid (HA) hydrogels containing IL-10, and poly(dl-lactide-co-glycolide) (PLGA)-HA nanoparticles loaded with ICA were incorporated into the composite hydrogels. The nanoparticle/hydrogel hybrid system demonstrates an array of features including mechanical strength, injectability and photo-crosslinking. The rapid release of IL-10 from the hydrogel effectively exerts immunomodulatory activity, whereas the long-term sustained release of icariin from the PLGA-HA nanoparticles significantly triggers the osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). Notably, the combined delivery of IL-10 and ICA from the hybrid system exhibit a synergistic effect for bone remodeling in a critical cranial defect rat model. Our findings indicate the importance of the immunomodulatory microenvironment and osteogenic differentiation for high-quality skull remodeling, and thus the dual-factor releasing nanoparticle/hydrogel hybrid system could be a promising candidate for repairing bone defects.
Keywords: Immunomodulation; Injectable hydrogel; Nanoparticles; Osteogenesis; Sequential release.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Shen X., Zhang Y., Gu Y., Xu Y., Liu Y., Li B., Chen L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials. 2016;106:205–216. - PubMed
-
- Wang J., Wu Y., Li G., Zhou F., Wu X., Wang M., Liu X., Tang H., Bai L., Geng Z., Song P., Shi Z., Ren X., Su J. Engineering large-scale self-mineralizing bone organoids with bone matrix-inspired hydroxyapatite hybrid bioinks. Adv. Mater. 2024 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

