The dynamics of prion spreading is governed by the interplay between the non-linearities of tissue response and replication kinetics
- PMID: 39717079
- PMCID: PMC11664133
- DOI: 10.1016/j.isci.2024.111381
The dynamics of prion spreading is governed by the interplay between the non-linearities of tissue response and replication kinetics
Abstract
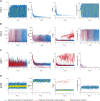
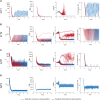
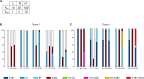
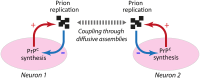
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are neurodegenerative disorders caused by the accumulation of misfolded conformers (PrPSc) of the cellular prion protein (PrPC). During the pathogenesis, the PrPSc seeds disseminate in the central nervous system and convert PrPC leading to the formation of insoluble assemblies. As for conventional infectious diseases, variations in the clinical manifestation define a specific prion strain which correspond to different PrPSc structures. In this work, we implemented the recent developments on PrPSc structural diversity and tissue response to prion replication into a stochastic reaction-diffusion model using an application of the Gillespie algorithm. We showed that this combination of non-linearities can lead prion propagation to behave as a complex system, providing an alternative to the current paradigm to explain strain-specific phenotypes, tissue tropisms, and strain co-propagation while also clarifying the role of the connectome in the neuro-invasion process.
Keywords: Biochemistry; Biocomputational method; Neuroscience.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

