Multifaceted virus-like particles: Navigating towards broadly effective influenza A virus vaccines
- PMID: 39717209
- PMCID: PMC11665419
- DOI: 10.1016/j.crmicr.2024.100317
Multifaceted virus-like particles: Navigating towards broadly effective influenza A virus vaccines
Abstract
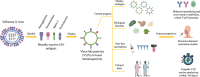
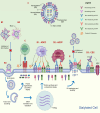
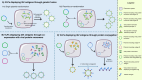
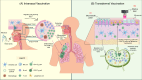
The threat of influenza A virus (IAV) remains an annual health concern, as almost 500,000 people die each year due to the seasonal flu. Current flu vaccines are highly dependent on embryonated chicken eggs for production, which is time consuming and costly. These vaccines only confer moderate protections in elderly people, and they lack cross-protectivity; thereby requiring annual reformulation to ensure effectiveness against contemporary circulating strains. To address current limitations, new strategies are being sought, with great emphasis given on exploiting IAV's conserved antigens for vaccine development, and by using different vaccine technologies to enhance immunogenicity and expedite vaccine production. Among these technologies, there are growing pre-clinical and clinical studies involving virus-like particles (VLPs), as they are capable to display multiple conserved IAV antigens and augment their immune responses. In this review, we outline recent findings involving broadly effective IAV antigens and strategies to display these antigens on VLPs. Current production systems for IAV VLP vaccines are comprehensively reviewed. Pain-free methods for administration of IAV VLP vaccines through intranasal and transdermal routes, as well as the mechanisms in stimulating immune responses are discussed in detail. The future perspectives of VLPs in IAV vaccine development are discussed, particularly concerning their potentials in overcoming current immunological limitations of IAV vaccines, and their inherent advantages in exploring intranasal vaccination studies. We also propose avenues to expedite VLP vaccine production, as we envision that there will be more clinical trials involving IAV VLP vaccines, leading to commercialization of these vaccines in the near future.
Keywords: Broadly reactive; Clinical trial; Conserved antigens; Influenza A virus; Pain-free; Production system; Vaccine; Virus-like particle.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A virus-like particle vaccine candidate for influenza A virus based on multiple conserved antigens presented on hepatitis B tandem core particles.Vaccine. 2018 Feb 1;36(6):873-880. doi: 10.1016/j.vaccine.2017.12.053. Epub 2018 Jan 3. Vaccine. 2018. PMID: 29306508
-
Protective CD8 T cell-mediated immunity against influenza A virus infection following influenza virus-like particle vaccination.J Immunol. 2013 Sep 1;191(5):2486-94. doi: 10.4049/jimmunol.1300954. Epub 2013 Jul 24. J Immunol. 2013. PMID: 23885108 Free PMC article.
-
Plant-derived virus-like particles as vaccines.Hum Vaccin Immunother. 2013 Jan;9(1):26-49. doi: 10.4161/hv.22218. Epub 2012 Sep 20. Hum Vaccin Immunother. 2013. PMID: 22995837 Free PMC article. Review.
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
Influenza Virus Like Particles (VLPs): Opportunities for H7N9 Vaccine Development.Viruses. 2020 May 8;12(5):518. doi: 10.3390/v12050518. Viruses. 2020. PMID: 32397182 Free PMC article. Review.
References
-
- AstraZeneca . 2022. FluenzⓇ Tetra product insert.https://www.ema.europa.eu/en/documents/product-information/fluenz-tetra-... (accessed 7 May 2024)
Publication types
LinkOut - more resources
Full Text Sources

