Evaluation and identification of metabolites produced by Cytobacillus firmus in the interaction with Arabidopsis thaliana plants and their effect on Solanum lycopersicum
- PMID: 39717210
- PMCID: PMC11665370
- DOI: 10.1016/j.crmicr.2024.100312
Evaluation and identification of metabolites produced by Cytobacillus firmus in the interaction with Arabidopsis thaliana plants and their effect on Solanum lycopersicum
Abstract
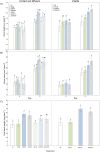
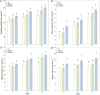
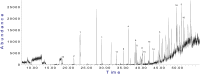
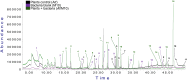
Currently, the use of bio-inputs is increasing due to the need to reduce the use of agrochemicals. However, one of the limitations is to preserve the viability of the living microorganisms, so it is important to find an alternative that allows us to obtain different metabolites to produce it. We evaluated three different interactions (contact, diffusible and volatile compounds) in vitro in Arabidopsis thaliana (At) seedlings with the strain Cytobacillus firmus M10 and its filtered secondary metabolites (M10F). The results showed that the seedlings inoculated by contact with the filtrate (AtM10F) presented increases in root length (30 %) and leaf area (33 %), as well as in the volatile interaction (At/M10F) with respect to the uninoculated treatment. For both interactions, the seedlings inoculated with the bacteria by contact (AtM10) and volatile (At/M10) obtained greater biomass (48 and 57 %). Subsequently, an evaluation at the end of the A. thaliana cycle showed that the treatments obtained by contact and distance when reinoculated with the bacteria and the filtrate (AtM10, At-M10 and AtM10F) obtained 50 % more seed yield than the control treatment, while AtM10F presented 72 %, while At/M10F presented the highest no. of siliques and seeds, which increased the yield by 65 %. In the Solanum lycopersicum (Sl) experiment, the filtrate (SlM10F) showed significant differences in seedling height, leaf length and width (23, 24 and 36 %, respectively). It also promoted an increase in fresh and dry weight, producing a greater root area and larger leaves compared to the control (Sl) and the bacteria (SlM10). We performed a qualitative characterization of the secondary metabolites present in the filtrate, where we found 2,4-DTBP, sylvopinol, isophthaladehyde, and eicosane of interest with possible growth-promoting effects on A. thaliana and tomato. We identified volatile compounds present in plant-microorganism and plant-filtrate interactions as possible precursors in the induction of plant growth, among which phenols, alcohols, aldehydes, alkanes, and alkenes stand out. Most of the analyzed compounds have not been found in the literature with reports of growth promoters, is important to mention that due to their characteristic functional groups they can derive and trigger the synthesis of new molecules with agronomic application.
Keywords: Diffusible compounds; Filtered metabolites; PGPRs; Plant-bacteria interaction; Plant-filtrate interaction; VOCs.
© 2024 Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Pablo Delgado Sanchez reports a relationship with Autonomous University of San Luis Potosi that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Erratum: High-Throughput Identification of Resistance to Pseudomonas syringae pv. Tomato in Tomato using Seedling Flood Assay.J Vis Exp. 2023 Oct 18;(200). doi: 10.3791/6576. J Vis Exp. 2023. PMID: 37851522
-
Priming of Plant Growth Promotion by Volatiles of Root-Associated Microbacterium spp.Appl Environ Microbiol. 2018 Oct 30;84(22):e01865-18. doi: 10.1128/AEM.01865-18. Print 2018 Nov 15. Appl Environ Microbiol. 2018. PMID: 30194105 Free PMC article.
-
Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth.Sci Total Environ. 2019 Nov 20;692:267-280. doi: 10.1016/j.scitotenv.2019.07.061. Epub 2019 Jul 5. Sci Total Environ. 2019. PMID: 31349168
-
Growth Promotion of Phaseolus vulgaris and Arabidopsis thaliana Seedlings by Streptomycetes Volatile Compounds.Plants (Basel). 2022 Mar 25;11(7):875. doi: 10.3390/plants11070875. Plants (Basel). 2022. PMID: 35406854 Free PMC article.
-
Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: Current understanding and future scope.Biotechnol Adv. 2023 Mar-Apr;63:108078. doi: 10.1016/j.biotechadv.2022.108078. Epub 2022 Dec 10. Biotechnol Adv. 2023. PMID: 36513315 Review.
References
-
- Abd-Elbaky A.A., Abo-Zaid G.A., Ahmed H.E.-F.M., Matar S.M., Abdel-Gayed M.A. Reducing the incidence of onion downy mildew disease using bio-formulation of Pseudomonas Fluorescens, limonene and acetyl salicylic acid. Plant Cell Biotechnol. Mol. Biol. 2021;22(1-2):103–120.
-
- Abdelkhalek A., Aseel D.G., Király L., Künstler A., Moawad H., Al-Askar A.A. Induction of systemic resistance to Tobacco mosaic virus in tomato through foliar application of Bacillus amyloliquefaciens Strain TBorg1 culture filtrate. Viruses. 2022;14(8):1830. doi: 10.3390/v14081830. - DOI - PMC - PubMed
-
- Adelusi O.A., Gbashi S., Adebiyi J.A., Makhuvele R., Adebo O.A., Aasa A.O., Targuma S., Kah G., Njobeh P.B. Variability in metabolites produced by Talaromyces pinophilus SPJ22 cultured on different substrates. Fungal. Bio. Biotechnol. 2022;9(1):15. doi: 10.1186/s40694-022-00145-8. - DOI - PMC - PubMed
-
- Adetutu A., Owoade A.O., Adegbola P.I. Inhibitory effects of ethyl acetate and butanol fractions from Morinda lucida benth on benzene-induced leukemia in mice. Arabian J. Chem. 2022;15(6) doi: 10.1016/j.arabjc.2022.103802. - DOI
-
- Agneeswari S., Amutha S., Jenishini J. Characterization and antimicrobial activity of Centella asiatica. Int. J. Eng. Adv. Technol. 2019;9:125–131. doi: 10.35940/ijeat.A1023.1291S619. - DOI
LinkOut - more resources
Full Text Sources

