Efficient synthesis of fluorinated triphenylenes with enhanced arene-perfluoroarene interactions in columnar mesophases
- PMID: 39717263
- PMCID: PMC11665444
- DOI: 10.3762/bjoc.20.270
Efficient synthesis of fluorinated triphenylenes with enhanced arene-perfluoroarene interactions in columnar mesophases
Abstract
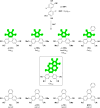
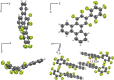
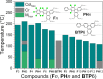
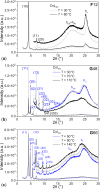
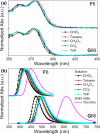
The high potential of non-covalent arene-fluoroarene intermolecular interactions in the design of liquid crystals lies in their ability to strongly promote self-assembly, improve the order and stability of the supramolecular mesophases, and enable tuneability of the optical and electronic properties, which can potentially be exploited for advanced applications in display technologies, photonic devices, sensors, and organic electronics. We recently successfully reported the straightforward synthesis of several mesogens containing four lateral aliphatic chains and derived from the classical triphenylene core self-assembling in columnar mesophases based on this paradigm. These mesogenic compounds were simply obtained in good yields by the nucleophilic substitution (SNFAr) of various types of commercially available fluoroarenes with the electrophilic organolithium derivatives 2,2'-dilithio-4,4',5,5'-tetraalkoxy-1,1'-biphenyl (2Li-BP n). In a continuation of this study, aiming at testing the limits of the reaction and providing a large diversity of structures, a structurally related series of compounds is reported here, namely 1,2,4-trifluoro-6,7,10,11-tetraalkoxy-3-(perfluorophenyl)triphenylenes (F n). They were obtained by reacting the above mentioned 2,2'-dilithiobiphenyl derivatives with decafluorobiphenyl, C6F5-C6F5. These compounds differ from the previously reported series, 1,2,4-trifluoro-6,7,10,11-tetraalkoxy-3-aryltriphenylenes (PH n), solely by the substitution of the terminal phenyl ring with a pentafluorophenyl ring. Thus, as expected, they display a Colhex mesophase over large temperature ranges, with only small differences in the mesophase stability and transition temperatures. Furthermore, the presence of the terminal fluorophenyl group enables a subsequent second annulation, yielding a new series of extended polyaromatic mesomorphic compounds, i.e., 1,1',3,3',4,4'-hexafluoro-6,6',7,7',10,10',11,11'-octaalkoxy-2,2'-bitriphenylene (G nm) which were found to display a Colrec mesophase. The specific nucleophilic substitution patterns of the F n derivatives and the antiparallel stacking mode into columnar structures stabilized by arene-perfluoroarene intermolecular interactions were confirmed by the single-crystal structure of the alkoxy-free side chain analog, i.e., 1,2,4-trifluoro-3-(perfluorophenyl)triphenylene (F). UV-vis absorption and fluorescence emission spectroscopies reveal green photoluminescence with fluorescence quantum yields of up to 33% for the F n derivatives. The J-aggregation for the inner fluorine-substituted dimers G nm is energetically and stereoelectronically more favorable and G66 exhibits thin-film fluorescence with a large red-shift of the emission peak.
Keywords: arene–perfluoroarene interaction; decafluorobiphenyl; fluorinated triphenylene; fluoroarene nucleophilic substitution; organolithium.
Copyright © 2024, Chen et al.
Figures



References
LinkOut - more resources
Full Text Sources
