Dual suppression of Glossina pallidipes using entomopathogenic fungal-based biopesticides and sterile insect technique
- PMID: 39717267
- PMCID: PMC11663849
- DOI: 10.3389/fmicb.2024.1472324
Dual suppression of Glossina pallidipes using entomopathogenic fungal-based biopesticides and sterile insect technique
Abstract
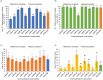
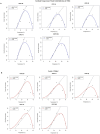
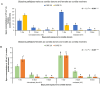
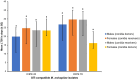
Tsetse flies and trypanosomosis significantly impact bovine production and human health in sub-Saharan Africa, exacerbating underdevelopment, malnutrition, and poverty. Despite various control strategies, long-term success has been limited. This study evaluates the combined use of entomopathogenic fungi (EPF) and the sterile insect technique (SIT) to combat tsetse flies. Eleven EPF isolates were tested against teneral males of Glossina pallidipes, focusing on mortality rates, radial growth, and impacts on fly fitness. Temperature effects on conidial growth, sporulation, and spore yield of SIT-compatible/tolerant strains were also assessed. The fungal isolates significantly influenced mortality rates in both unirradiated and irradiated (SIT-treated) males (p < 0.0001). Metarhizium anisopliae strains ICIPE 20, ICIPE 32, ICIPE 41, ICIPE 62, ICIPE 78, and Beauveria bassiana ICIPE 603 showed higher SIT compatibility/tolerance with LT50 values of 11-30 days, compared to other more virulent isolates with LT50 values of 4-9 days. Temperature significantly affected the radial growth of SIT-compatible EPF strains (p < 0.0001), with M. anisopliae ICIPE 78 exhibiting the fastest conidia growth at 25°C. Spore yield varied significantly across temperatures (15-40°C), and the thermal range for conidia germination of SIT-compatible strains was 8.1-45.4°C, with an optimal range of 26.7-31.1°C. Moreover, infected unirradiated females and irradiated males (donors) successfully transmitted conidia to untreated flies (receivers) without significant differences in survival rates (p = 0.6438) and no observed sex dimorphism. Our findings highlight the potential of combining EPF and SIT as a novel dual approach that could effectively and synergistically suppress tsetse fly populations.
Keywords: African animal trypanosomiasis (AAT); Beauveria bassiana; Glossina pallidipes; Metarhizium anisopliae; area-wide integrated pest management (AW-IPM); human African trypanosomiasis (HAT).
Copyright © 2024 Ombura, Abd-Alla, Akutse, Runo, Mireji, Bateta, Otiwi, Ajene and Khamis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abbott W. S. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265a - DOI
-
- Agbessenou A., Akutse K. S., Yusuf A. A., Wekesa S. W., Khamis F. M. (2021). Temperature-dependent modelling and spatial prediction reveal suitable geographical areas for deployment of two Metarhizium anisopliae isolates for Tuta absoluta management. Sci. Rep. 11:23346. doi: 10.1038/s41598-021-02718-w, PMID: - DOI - PMC - PubMed
-
- Akutse K. S., Subramanian S., Maniania N., Dubois T., Ekesi S. (2020). Biopesticide research and product development in Africa for sustainable agriculture and food security–experiences from the International Centre of Insect Physiology and Ecology (icipe). Front. Sustain. Food Syst. 4:152. doi: 10.3389/fsufs.2020.563016 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

