ACE Loss Drives Renal Cell Carcinoma Growth and Invasion by Modulating AKT-FOXO1
- PMID: 39717370
- PMCID: PMC11665188
- DOI: 10.2147/BTT.S485178
ACE Loss Drives Renal Cell Carcinoma Growth and Invasion by Modulating AKT-FOXO1
Abstract
Purpose: Emerging literature links the role of the renin-angiotensin-aldosterone system (RAAS) to the progression of cancers. However, the function of RAAS has not been verified in Clear-cell renal cell carcinoma (ccRCC).
Methods: ACE expression in ccRCC tissues was determined using RT-PCR, Western blot, and immunohistochemistry staining. The clinical significance of ACE was evaluated through Cox regression analysis. To assess the impact of ACE expression on ccRCC cell growth, metastasis, and glucose activity, CCK-8 assays, transwell assays, Seahorse detection, and xenograft models were utilized. The mechanisms of ACE and its upstream and downstream regulatory factors were investigated using RNA-seq, chromatin immunoprecipitation (ChIP), and luciferase reporter assays.
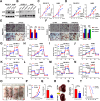
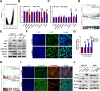
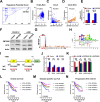
Results: RAAS-related gene Angiotensin-Converting Enzyme (ACE) was significantly under expressed in ccRCC cells and tissues. High ACE expression was positively associated with a favorable prognosis in ccRCC patients. Functional studies showed that ACE overexpression suppressed ccRCC cell line OS-RC-2 and A498 growth, metastasis, and glycolysis activities, while its knockdown had the opposite effect. Mechanistically, ACE inhibited ccRCC progression and epithelial-mesenchymal transition (EMT) by disrupting the AKT-FOXO1 signaling pathway. Furthermore, we provide evidence that ACE could enhance everolimus (approved agent for ccRCC) antitumor effect and ACE expression is transcriptionally regulated by ZBTB26.
Conclusion: Our findings investigated the roles and mechanisms of ACE in ccRCC. ACE inhibits the growth and metastasis of ccRCC cells in vitro and in vivo by promoting FOXO1 expression, which is the downstream target of PI3K-AKT pathway. Thus, this research suggests that ACE may be a promising target for new therapeutic strategy in ccRCC.
Keywords: ACE; AKT; FOXO1; clear-cell renal cell carcinoma; everolimus.
© 2024 Yin et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

