The Biological Effects of Magnesium-Based Implants on the Skeleton and Their Clinical Implications in Orthopedic Trauma Surgery
- PMID: 39717475
- PMCID: PMC11665827
- DOI: 10.34133/bmr.0122
The Biological Effects of Magnesium-Based Implants on the Skeleton and Their Clinical Implications in Orthopedic Trauma Surgery
Abstract
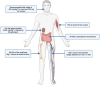
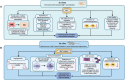
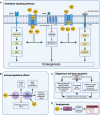
Magnesium (Mg)-based implants have evolved as a promising innovation in orthopedic trauma surgery, with the potential to revolutionize the treatment of bone diseases, including osteoporotic fractures and bone defects. Available clinical studies mostly show excellent patient outcomes of resorbable Mg-based implants, without the need for subsequent implant removal. However, the occurrence of radiolucent zones around Mg-based implants seems to be a noticeable drawback for a more widespread clinical use. Mechanistically, both in vivo and in vitro studies demonstrated beneficial effects on the formation of new bone, a unique characteristic of Mg-based implants. In this regard, Mg has been shown to exert pleiotropic functions on osteogenic differentiation and migration of osteoblasts and their precursors. Additionally, collective evidence suggests that Mg-based implants promote angiogenesis in newly formed bone and exert immunomodulatory effects in the bone microenvironment. Likewise, Mg-based implants and their degradation products were shown to inhibit bone resorption by impairing osteoclastogenesis. The purpose of this review is to provide a state-of-the-art summary of the clinical and basic science evidence regarding the performance of currently used Mg-based implants. In addition to the status of in vivo and in vitro research and clinical applications, future challenges and perspectives of Mg-based orthopedic implants are discussed.
Copyright © 2024 Elena Müller et al.
Conflict of interest statement
Competing interests: J.-M.S. and A.K. are employees of Medical Magnesium GmbH and Meotec GmbH. The other authors declare no competing interests.
Figures
Similar articles
-
Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study.Acta Biomater. 2018 Jan;65:486-500. doi: 10.1016/j.actbio.2017.10.033. Epub 2017 Oct 25. Acta Biomater. 2018. PMID: 29079514
-
Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives.Adv Sci (Weinh). 2020 Feb 28;7(8):1902443. doi: 10.1002/advs.201902443. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32328412 Free PMC article. Review.
-
Versatile application of magnesium-related bone implants in the treatment of bone defects.Mater Today Bio. 2025 Mar 5;31:101635. doi: 10.1016/j.mtbio.2025.101635. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124334 Free PMC article. Review.
-
Comparison of a resorbable magnesium implant in small and large growing-animal models.Acta Biomater. 2018 Sep 15;78:378-386. doi: 10.1016/j.actbio.2018.07.044. Epub 2018 Jul 29. Acta Biomater. 2018. PMID: 30059798
-
Development of degradable magnesium-based metal implants and their function in promoting bone metabolism (A review).J Orthop Translat. 2022 Oct 8;36:184-193. doi: 10.1016/j.jot.2022.09.013. eCollection 2022 Sep. J Orthop Translat. 2022. PMID: 36263386 Free PMC article. Review.
References
-
- Manam NS, Harun WSW, Shri DNA, Ghani SAC, Kurniawan T, Ismail MH, Ibrahim MHI. Study of corrosion in biocompatible metals for implants: A review. J Alloys Compd. 2017;701:698–715.
Publication types
LinkOut - more resources
Full Text Sources

