Biliary atresia susceptibility gene EFEMP1 regulates extrahepatic bile duct elastic fiber formation and mechanics
- PMID: 39717503
- PMCID: PMC11663959
- DOI: 10.1016/j.jhepr.2024.101215
Biliary atresia susceptibility gene EFEMP1 regulates extrahepatic bile duct elastic fiber formation and mechanics
Abstract
Background & aims: EGF-containing fibulin extracellular matrix protein 1 (EFEMP1, also called fibulin-3) is an extracellular matrix protein linked in a genome-wide association study to biliary atresia, a fibrotic disease of the neonatal extrahepatic bile duct. Fibulin-3 is deposited in most tissues and Efemp1 null mice have decreased elastic fibers in visceral fascia; however, fibulin-3 does not have a role in the development of large elastic fibers and its overall function in the extrahepatic bile ducts remains unclear.
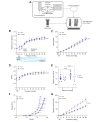
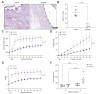
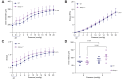
Methods: We used staining and histology to define the amount and organization of key extracellular matrix components in the extrahepatic bile ducts. We also repurposed pressure myography, a technique heretofore applied to the vasculature, to determine the contribution of elastin and fibulin-3 to extrahepatic bile duct mechanics. We examined extrahepatic bile duct structure and mechanics in three models: neonatal vs. adult rat ducts (n = 6 each), elastase-treated adult rat ducts (n = 6-7 each), and Efemp1 +/- vs. wild-type mouse ducts (n = 6 each).
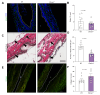
Results: We demonstrated that fibulin-3 is expressed in the submucosa of both neonatal and adult mouse, rat and human extrahepatic bile ducts and that, in adult Efemp1 +/- mouse ducts, elastin organization into fibers is decreased by approximately half. Pressure myography showed that Efemp1 +/- ducts have altered mechanics compared to control ducts, with Efemp1 +/- ducts displaying significant stretch compared to controls (p = 0.0376); these changes in stretch are similar to those observed in elastase-treated vs. normal ducts (p <0.0001) and in neonatal ducts vs. adult ducts (p <0.0001).
Conclusion: Fibulin-3 has an important role in the formation of elastic fibers and the mechanical properties of the extrahepatic bile duct. This provides functional relevance for the biliary atresia susceptibility gene EFEMP1.
Impact and implications: The gene EFEMP1 was found via a genome-wide association study to be a susceptibility gene for the neonatal disease biliary atresia. EFEMP1 encodes the protein fibulin-3, which regulates elastic fiber organization in the extrahepatic bile duct (EHBD), the major site of disease in biliary atresia. We showed that neonatal EHBDs as well as mice heterozygous for Efemp1 have decreased numbers of elastic fibers, and that this alters EHBD mechanics. This work is important for understanding the mechanism of biliary atresia, in particular susceptibility to obstruction.
Keywords: Pressure myography; bile duct mechanics; collagen; elastin; mechanobiology; stiffness.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


Similar articles
-
Microcystin-RR is a biliary toxin selective for neonatal extrahepatic cholangiocytes.JHEP Rep. 2024 Sep 12;7(1):101218. doi: 10.1016/j.jhepr.2024.101218. eCollection 2025 Jan. JHEP Rep. 2024. PMID: 39687604 Free PMC article.
-
Glucocorticosteroids for infants with biliary atresia following Kasai portoenterostomy.Cochrane Database Syst Rev. 2018 May 14;5(5):CD008735. doi: 10.1002/14651858.CD008735.pub3. Cochrane Database Syst Rev. 2018. PMID: 29761473 Free PMC article.
-
PTEN deficiency induces an extrahepatic cholangitis-cholangiocarcinoma continuum via aurora kinase A in mice.J Hepatol. 2024 Jul;81(1):120-134. doi: 10.1016/j.jhep.2024.02.018. Epub 2024 Feb 28. J Hepatol. 2024. PMID: 38428643 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Pathological Changes in Extracellular Matrix Composition Orchestrate the Fibrotic Feedback Loop Through Macrophage Activation in Dupuytren's Contracture.Int J Mol Sci. 2025 Mar 28;26(7):3146. doi: 10.3390/ijms26073146. Int J Mol Sci. 2025. PMID: 40243889 Free PMC article.
-
Genetic background and biliary atresia.World J Pediatr Surg. 2025 Jun 6;8(3):e001023. doi: 10.1136/wjps-2025-001023. eCollection 2025. World J Pediatr Surg. 2025. PMID: 40519535 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

