Distinct virologic trajectories in chronic hepatitis B identify heterogeneity in response to nucleos(t)ide analogue therapy
- PMID: 39717508
- PMCID: PMC11664071
- DOI: 10.1016/j.jhepr.2024.101229
Distinct virologic trajectories in chronic hepatitis B identify heterogeneity in response to nucleos(t)ide analogue therapy
Abstract
Background & aims: The dynamics of HBV viral load (VL) in patients with chronic hepatitis B (CHB) on nucleos(t)ide analogue (NA) treatment and its relationship with liver disease are poorly understood. We aimed to study longitudinal VL patterns and their associations with CHB clinical outcomes.
Methods: Utilising large scale, routinely collected electronic health records from six centres in England, collated by the National Institute for Health and Care Research Health Informatics Collaborative (NIHR HIC), we applied latent class mixed models to investigate VL trajectory patterns in adults receiving NA treatment. We assessed associations of VL trajectory with alanine transaminase, and with liver fibrosis/cirrhosis.
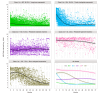
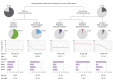
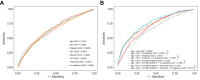
Results: We retrieved data from 1,885 adults on NA treatment (median follow-up 6.2 years, IQR 3.7-9.3 years), with 21,691 VL measurements (median 10 per patient, IQR 5-17). Five VL classes were identified from the derivation cohort (n = 1,367, discrimination: 0.93, entropy: 0.90): class 1 'long term suppression' (n = 827, 60.5%), class 2 'timely virological suppression' (n = 254, 18.6%), class 3 'persistent moderate viraemia' (n = 140, 10.2%), class 4 'persistent high-level viraemia' (n = 44, 3.2%), and class 5 'slow virological suppression' (n = 102, 7.5%). The model demonstrated a discrimination of 0.93 and entropy of 0.88 for the validation cohort (n = 518). Alanine transaminase decreased variably over time in VL-suppressed groups (classes 1, 2, 5; all p <0.001), but did not significantly improve in those with persistent viraemia (classes 3, 4). Patients in class 5 had twofold increased hazards of fibrosis/cirrhosis compared with class 1 (adjusted hazard ratio, 2.00; 95% CI, 1.33-3.02).
Conclusions: Heterogeneity exists in virological response to NA therapy in CHB patients, with over 20% showing potentially suboptimal responses. Slow virological suppression is associated with liver disease progression.
Impact and implications: Treatment recommendations for people living with chronic hepatitis B virus (HBV) infection are becoming less stringent, meaning that more of the population will be eligible to receive therapy with nucleos(t)ide analogue agents. We explored outcomes of HBV treatment in a large UK dataset, describing different responses to treatment, and showing that the viral load is not completely suppressed after 1 year in about one in five cases, associated with an increased risk of liver complications. As treatment is rolled out more widely, patients and clinicians need to be aware of the potential for incomplete virologic responses. The findings can support the identification of high-risk individuals, improve early fibrosis and cirrhosis prediction, guide monitoring and preventive interventions, and support public health elimination goals.
Keywords: Antiviral treatment; Cirrhosis; HBV; Health Informatics Collaborative (HIC); Latent class mixed models; Liver fibrosis; Longitudinal; Nucleotide analogue; Viral load.
© 2024 The Author(s).
Conflict of interest statement
GC reports personal fees from Gilead and Merck Sharp & Dohme, outside the submitted work. ST reports she has previously received Gilead Investigator-led grant for a viral hepatitis project. WG reports personal fees from GSK outside the submitted work. EB has consulted for, and received research grants from Roche and GSK. EB and PCM have academic collaborative partnerships with GSK. Other authors have no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Hsu Y.C., Huang D.Q., Nguyen M.H. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524–537. - PubMed
-
- World Health Organization Hepatitis B 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
-
- World Health Organization . WHO; Geneva: 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections.
-
- World Health Organization . WHO; Geneva: 2024. Global hepatitis report 2024 action for access in low- and middle-income countries.
-
- World Health Organization Global health sector strategy on viral hepatitis 2016-2021. Available from: 2016. https://apps.who.int/iris/handle/10665/246177
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

