vCPP2319 interacts with metastatic breast cancer extracellular vesicles (EVs) and transposes a human blood-brain barrier model
- PMID: 39717586
- PMCID: PMC11664409
- DOI: 10.1016/j.heliyon.2024.e40907
vCPP2319 interacts with metastatic breast cancer extracellular vesicles (EVs) and transposes a human blood-brain barrier model
Abstract
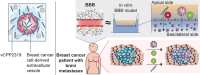
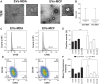
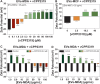
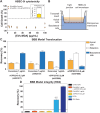
Brain metastases (BM) are frequently found in cancer patients and, though their precise incidence is difficult to estimate, there is evidence for a correlation between BM and specific primary cancers, such as lung, breast, and skin (melanoma). Among all these, breast cancer is the most frequently diagnosed among women and, in this case, BM cause a critical reduction of the overall survival (OS), especially in triple negative breast cancer (TNBC) patients. The main challenge of BM treatment is the impermeable nature of the blood-brain barrier (BBB), which shields the central nervous systems (CNS) from chemotherapeutic drugs. Extracellular vesicles (EVs) have been proposed as ideal natural drug carriers and these may exhibit some advantages over synthetic nanoparticles (NPs). In this work, we isolate breast cancer-derived EVs and study their ability to carry vCPP2319, a peptide with dual cell-penetration and anticancer activities. The selective cytotoxicity of anticancer peptide-loaded EVs towards breast cancer cells and their ability to translocate an in vitro BBB model are also addressed. Overall, it was possible to conclude that vCPP2319 naturally interacts with breast cancer-derived EVs, being retained at the surface of these vesicles. Moreover, the results revealed a cytotoxic activity for peptide-loaded EVs similar to that obtained with the peptide alone and the ability of peptide-loaded EVs to translocate an in vitro BBB model, which contrasts with the results obtained with the peptide alone. In conclusion, this work supports the use of EVs in the development of biological drug-delivery systems (DDS) capable of translocating the BBB.
Keywords: Anticancer peptide; Blood-brain barrier; Brain metastases; Drug-delivery systems; Extracellular vesicles; Metastatic breast cancer.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
LinkOut - more resources
Full Text Sources

