Latitudinal Clines in an Ectothermic Vertebrate: Patterns in Body Size, Growth Rate, and Reproductive Effort Suggest Countergradient Responses in the Prairie Lizard
- PMID: 39717636
- PMCID: PMC11664205
- DOI: 10.1002/ece3.70680
Latitudinal Clines in an Ectothermic Vertebrate: Patterns in Body Size, Growth Rate, and Reproductive Effort Suggest Countergradient Responses in the Prairie Lizard
Abstract
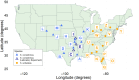
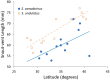
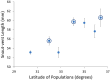
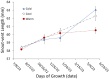
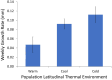
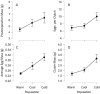
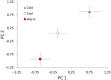
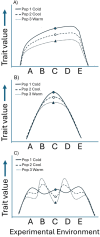
Although we have evidence that many organisms are exhibiting declines in body size in response to climate warming, we have little knowledge of underlying mechanisms or how associated phenotypic suites may coevolve. The better we understand coadaptations among physiology, morphology, and life history, the more accurate our predictions will be of organismal response to changing thermal environments. This is especially salient for ectotherms because they comprise 99% of species worldwide and are key to functioning ecosystems. Here, we assess body size, growth rates, and reproductive traits of a vertebrate ectotherm, the prairie lizard, Sceloporus consobrinus, for multiple populations along a latitudinal thermal gradient and compare body size clines between S. consobrinus and eastern fence lizard (S. undulatus) populations. We found that phenotypic values increased as environmental temperatures decreased for all traits examined, resulting in a pattern representative of countergradient variation. The positive covariation of phenotypes across the thermal gradient exemplifies the enigma of "master of all traits." This enigma was further illustrated by the energy expenditure toward growth and reproduction increasing as phenotypic values increased. The evolutionary responses appear to reveal overcompensation because annual energy expenditure toward growth and reproduction increased even as activity periods decreased. Overall, compensatory responses to cooler thermal environments were exhibited by prairie lizards in body size, growth rate, egg size, and clutch size, resulting in cold-adapted populations allocating more energy toward maintenance, growth, and reproduction than lower latitude, warm-adapted populations. If larger body size in ectotherms is a result of intrinsically faster physiological rates compensating for cooler temperatures and shorter activity periods, then smaller body sizes in warmer environments may be a result of greater reliance on available environmental temperatures for physiological rates and time for assimilating resources.
Keywords: Bergmann's cline; Sceloporus consobrinus; energy budgets; life history; thermal adaptations.
© 2024 The Author(s). Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Allen, J. A. 1877. “The Influence of Physical Conditions in the Genesis of Species.” Radical Review 1: 108–140.
-
- Andrew, N. R. , and Hughes L.. 2005. “Arthropod Community Structure Along a Latitudinal Gradient: Implications for Future Impacts of Climate Change.” Austral Ecology 30: 281–297. 10.1111/j.1442-9993.2005.01464.x. - DOI
-
- Andrews, R. M. 1998. “Geographic Variation in Field Body Temperature of Sceloporus Lizards.” Journal of Thermal Biology 23, no. 6: 329–334.
-
- Angilletta, M. J., Jr. 2001. “Variation in Metabolic Rate Between Populations of a Geographically Widespread Lizard.” Physiological and Biochemical Zoology 74, no. 1: 11–21. - PubMed
-
- Angilletta, M. J., Jr. , Niewiarowski P. H., Dunham A. E., Leaché A. D., and Porter W. P.. 2004. “Bergmann's Clines in Ectotherms: Illustrating a Life‐History Perspective With Sceloporine Lizards.” American Naturalist 164, no. 6: E168–E183. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

