Phylogenetic diversity and morphological characterization of cordycipitaceous species in Taiwan
- PMID: 39717658
- PMCID: PMC11663429
- DOI: 10.3114/sim.2024.109.01
Phylogenetic diversity and morphological characterization of cordycipitaceous species in Taiwan
Abstract
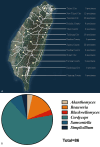
Species classified in Cordycipitaceae (Hypocreales) include multiple entomopathogenic fungi. Numerous changes have recently occurred in the nomenclature of cordycipitaceous fungi due to the single naming system proposed for pleomorphic fungi in 2011. Species of Cordycipitaceae are widely applied as herbal medicines, especially in Asian cultures. However, the diversity of Cordycipitaceae in Taiwan is based on relatively few literature records. Here we conducted a comprehensive survey of this family throughout the island of Taiwan and provided a glimpse of the diversity and distribution patterns. In addition, the present study reassesses the generic and species boundaries of Cordycipitaceae and finally provides an updated phylogenetic overview of Cordyceps and allied genera. Phylogenetic reconstructions using combined ITS, nrLSU, tef1-α, rpb1, and rpb2 sequence data, along with morphological features, resulted in 10 novel species: Akanthomyces taiwanicus sp. nov., Blackwellomyces taiwanensis sp. nov., Cordyceps hehuanensis sp. nov., C. locastrae sp. nov., C. malleiformis sp. nov., C. pseudorosea sp. nov., C. siangyangensis sp. nov., Samsoniella lasiocampidarum sp. nov., S. yuanzuiensis sp. nov., and Simplicillium salviniae sp. nov.; and nine new records for Taiwan: A. kanyawimiae, A. muscarius, S. cardinalis, S. hepiali, B. lii, B. medogensis, C. lepidopterorum, C. neopruinosa, and Si. chinense. Furthermore, we provided DNA sequence data of the ex-type strains of C. ninchukispora for the first time and determined the species limits of the taxon. In addition, the present study proposed to synonymize B. staphylinidicola and C. jakajanicola under B. bassiana and C. lepidopterorum, respectively. Moreover, three species, C. roseostromata, C. kyushuensis, and C. shuifuensis, that clustered within the species clade of C. militaris are proposed to be synonymized under the latter taxon. To maintain the monophyly of Cordyceps, we propose to classify Parahevansia koratensis in Cordyceps, which makes the genus Parahevansia obsolete. Taxonomic novelties: New species: Akanthomyces taiwanicus W.Y. Chuang, B. Shrestha & H.A. Ariyaw., Blackwellomyces taiwanensis W.Y. Chuang & H.A. Ariyaw., Cordyceps hehuanensis W.Y. Chuang & H.A. Ariyaw., C. locastrae W.Y. Chuang & H.A. Ariyaw., C. malleiformis W.Y. Chuang & H.A. Ariyaw., C. pseudorosea W.Y. Chuang & H.A. Ariyaw., C. siangyangensis W.Y. Chuang & H.A. Ariyaw., Samsoniella lasiocampidarum W.Y. Chuang & H.A. Ariyaw., S. yuanzuiensis W.Y. Chuang & H.A. Ariyaw., Simplicillium salviniae W.Y. Chuang & H.A. Ariyaw. New combination: Cordyceps koratensis (Hywel-Jones) H.A. Ariyaw., M. Stadler & Luangsa-ard. New synonyms: Beauveria bassiana (Bals.-Criv.) Vuill., Cordyceps lepidopterorum Mongkols. et al., C. militaris (L.) Fr. Citation: Chuang WY, Lin YC, Shrestha B, Luangsa-ard JJ, Stadler M, Tzean SS, Wu S, Ko CC, Hsieh SY, Wu ML, Wang SC, Shen TL, Ariyawansa HA (2024). Phylogenetic diversity and morphological characterization of cordycipitaceous species in Taiwan. Studies in Mycology 109: 1-56. doi: 10.3114/sim.2024.109.01.
Keywords: Cordycipitaceae; entomopathogenic fungi; new species; phylogenetic analyses.
© 2024 Westerdijk Fungal Biodiversity Institute.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




























References
-
- Araújo JP, Hughes DP. (2016). Diversity of entomopathogenic fungi: which groups conquered the insect body? Advances in Genetics 94: 1–39. - PubMed
-
- Ariyawansa HA, Hyde KD, Jayasiri SC. et al. (2015). Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75: 27–274.
-
- Chen CH, Hsieh SY, Yeh YH. et al. (2020). Cladocillium musae, a new genus and species of cercosporoid fungi (Mycosphaerellaceae) on wild banana in Taiwan. Mycological Progress 19: 837–843.
LinkOut - more resources
Full Text Sources
Research Materials
