Efficacy and safety of hypomethylating agents in the treatment of AML/MDS patients relapsed post allogenetic hematopoietic stem cell transplantation
- PMID: 39717745
- PMCID: PMC11663890
- DOI: 10.3389/fonc.2024.1465334
Efficacy and safety of hypomethylating agents in the treatment of AML/MDS patients relapsed post allogenetic hematopoietic stem cell transplantation
Abstract
Introduction: Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) constitute myeloid malignancies, and allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered as a potentially optimal approach for achieving a long term cure. However, post-allo-HSCT relapse remains a leading cause of mortality and therapeutic failure.
Methods: To evaluate the efficacy and safety of combining hypomethylating agents (HMAs) with Bcl-2 inhibitors in the treatment of AML/MDS relapse following allo-HSCT, we retrospectively collected data from 42 patients who experienced relapse between April 2012 and March 2022 at Peking University First Hospital. Among these patients, 21 underwent intensive chemotherapy (IC) alone, while the other 21 received treatment with HMAs after IC treatment, either alone or in combination with the Bcl-2 inhibitor venetoclax (VEN).
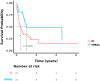
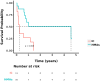
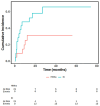
Results: The median overall survival (OS) was 9 ± 2.153 months, and the one-year OS rate was 41.5%. The overall response rate (ORR) in the chemotherapy group and the IC+HMAs ± VEN group was 52.38% (11/21) and 76.19% (16/21), respectively, with no significant difference found (P=0.107). Kaplan-Meier analysis revealed a significant difference in OS between the chemotherapy group and the IC+HMAs ± VEN group in our retrospective cohort study (P=0.041, χ2= 4.016). Additionally, a significant difference in overall survival (OS) rates was observed between the two groups for patients categorized as intermediate/high risk (P=0.008). The secondary relapse rate was 45.45% (5/11) in the IC cohort and 25% (4/16) in the IC+HMAs ± VEN group, respectively, with no significant difference identified between the two cohorts (P=0.268). Furthermore, upon assessing the risk of graft-versus-host disease (GvHD), infection, and agranulocytosis, no notable differences were observed with the use of HMAs, suggesting that HMAs did not increase the risk. In the IC+HMAs ± VEN group, 7 patients received VEN in addition to HMAs, and no significant statistical difference was found in OS when comparing patients who received HMAs alone and those who received HMA+VEN (P=0.183), also, a statistically significant difference in OS was noted between the two groups whenaccounting for competing risks (P=0.028).
Conclusions: This retrospective study highlights the efficacy of IC+HMAs ± VEN in treating AML/MDS patients experiencing relapse post allo-HSCT, improving survival rates, especially for those classified as intermediate/high risk, with favorable tolerability.
Keywords: allo-HSCT; hypomethylating agents; myeloid malignancy; relapse; venetoclax.
Copyright © 2024 Wang, Wang, Ren, Dong, Wang, Liang, Yin, Liu, Xu, Han and Li.
Conflict of interest statement
The authors assert that the research was carried out without any commercial or financial relationships that could be perceived as a potential conflict of interest.
Figures



Similar articles
-
Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome.Cancer Sci. 2021 Sep;112(9):3636-3644. doi: 10.1111/cas.15048. Epub 2021 Jul 21. Cancer Sci. 2021. PMID: 34185931 Free PMC article.
-
Venetoclax plus hypomethylating agents versus intensive chemotherapy for hematological relapse of myeloid malignancies after allo-HSCT.Front Oncol. 2023 Mar 23;13:1137175. doi: 10.3389/fonc.2023.1137175. eCollection 2023. Front Oncol. 2023. PMID: 37035180 Free PMC article.
-
Outcome after allogeneic hematopoietic stem cell transplantation following Venetoclax-based therapy among AML and MDS patients.Ann Hematol. 2022 Dec;101(12):2731-2741. doi: 10.1007/s00277-022-04983-9. Epub 2022 Nov 1. Ann Hematol. 2022. PMID: 36318288
-
Venetoclax and hypomethylating agents versus induction chemotherapy for newly diagnosed acute myeloid leukemia patients: a systematic review and meta-analysis.BMC Cancer. 2025 May 19;25(1):894. doi: 10.1186/s12885-025-14311-9. BMC Cancer. 2025. PMID: 40389911 Free PMC article.
-
Hypomethylating Agents and FLT3 Inhibitors As Maintenance Treatment for Acute Myeloid Leukemia and Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation-A Systematic Review and Meta-Analysis.Transplant Cell Ther. 2021 Dec;27(12):997.e1-997.e11. doi: 10.1016/j.jtct.2021.09.005. Epub 2021 Sep 20. Transplant Cell Ther. 2021. PMID: 34551341 Free PMC article.
Cited by
-
Drug-tolerant persister cells in acute myeloid leukemia: pressing challenge and promising new strategies for treatment.Front Med (Lausanne). 2025 May 14;12:1586552. doi: 10.3389/fmed.2025.1586552. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40443513 Free PMC article. Review.
References
-
- Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. . Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007 - DOI - PMC - PubMed
-
- Ciurea SO, Labopin M, Socie G, Volin L, Passweg J, Chevallier P, et al. . Relapse and survival after transplantation for complex karyotype acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation and the University of Texas MD Anderson Cancer Center. Cancer. (2018) 124(10):2134–41. doi: 10.1002/cncr.31311 - DOI - PubMed
-
- Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. . Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. (2007) 25(31):4938–45. doi: 10.1200/JCO.2007.11.6053 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

