Catalyzing Pharmacogenomic Analysis for Informing Pain Treatment (C-PAIN): A Randomized Trial of Preemptive CYP2D6 Genotyping in Cancer Palliative Care
- PMID: 39717756
- PMCID: PMC11664000
- DOI: 10.2147/JPR.S488416
Catalyzing Pharmacogenomic Analysis for Informing Pain Treatment (C-PAIN): A Randomized Trial of Preemptive CYP2D6 Genotyping in Cancer Palliative Care
Abstract
Background: Cancer patients frequently suffer from pain, often managed with opioids. However, undertreated pain remains a significant concern. Opioid effectiveness varies due to genetic differences in how individuals metabolize some of these medications. While prior research suggests promise in tailoring opioid prescriptions based on CYP2D6 genetic makeup, its application in cancer pain management remains limited. This study investigates the potential benefits of preemptive CYP2D6 genotyping for cancer patients initiating opioid therapy, focusing on codeine, tramadol, and hydrocodone, whose efficacy is demonstrably impacted by CYP2D6 variations.
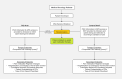
Methods: This is a randomized, prospective study to evaluate the effects of preemptive pharmacogenomic (PGx) testing on opioid dosing decisions/selections and composite pain score in oncology patients. Patients with metastatic solid tumors for whom near-future opioid therapy is anticipated will be randomized to PGx and control arms, stratified by the presence or absence of bony metastases and history of opioid use. In the PGx arm, patients will be preemptively tested using a panel of pharmacogenomic genetic variants, and providers will receive opioid dosing guidance via an electronic medical record-embedded clinical decision support tool. In the control arm, pain prescribing will occur per standard of care without genotype information.
Planned outcome: The primary study outcome will be composite pain intensity during the first 45 days after an index opioid prescription for codeine, tramadol, or hydrocodone. Safety will be assessed by comparing opioid-related adverse event rates between the two study arms. Secondary outcomes will include rates of hospitalization/emergency room visits, cumulative morphine equivalents received, and type of first opioid prescribed.
Keywords: CYP2D6; codeine; genetic testing; hydrocodone; pain score; pharmacogenomics; tramadol.
© 2024 Cho et al.
Conflict of interest statement
Dr Russell Szmulewitz reports grants from Bayer, grants from Pfizer, personal fees from Novartis, outside the submitted work. Dr Everett Vokes reports personal fees from AbbVie, personal fees, non-financial support from AstraZeneca, personal fees from BioNTech, outside the submitted work. Dr Mark Ratain reports grants from National Institutes of Health, during the conduct of the study. Dr Peter O’Donnell reports grants from National Institutes of Health, during the conduct of the study; personal fees from National Institutes of Health, personal fees from O’Brien and Ryan LLP, personal fees from ISMIE, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Impact of CYP2D6 Pharmacogenomic Status on Pain Control Among Opioid-Treated Oncology Patients.Oncologist. 2021 Nov;26(11):e2042-e2052. doi: 10.1002/onco.13953. Epub 2021 Sep 19. Oncologist. 2021. PMID: 34423496 Free PMC article.
-
Implementing a pragmatic clinical trial to tailor opioids for acute pain on behalf of the IGNITE ADOPT PGx investigators.Clin Transl Sci. 2022 Oct;15(10):2479-2492. doi: 10.1111/cts.13376. Epub 2022 Aug 4. Clin Transl Sci. 2022. PMID: 35899435 Free PMC article. Clinical Trial.
-
A Randomized Hybrid-Effectiveness Trial Comparing Pharmacogenomics (PGx) to Standard Care: The PGx Applied to Chronic Pain Treatment in Primary Care (PGx-ACT) Trial.Clin Transl Sci. 2025 Feb;18(2):e70154. doi: 10.1111/cts.70154. Clin Transl Sci. 2025. PMID: 39921243 Free PMC article. Clinical Trial.
-
The Role of Pharmacogenomics in Opioid Prescribing.Curr Treat Options Oncol. 2022 Oct;23(10):1353-1369. doi: 10.1007/s11864-022-01010-x. Epub 2022 Aug 24. Curr Treat Options Oncol. 2022. PMID: 36001223 Free PMC article. Review.
-
Clinical Implications of Opioid Pharmacogenomics in Patients With Cancer.Cancer Control. 2015 Oct;22(4):426-32. doi: 10.1177/107327481502200408. Cancer Control. 2015. PMID: 26678969 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

