Investigating the crosstalk between ABCC4 and ABCC5 in 3T3-L1 adipocyte differentiation
- PMID: 39717760
- PMCID: PMC11663720
- DOI: 10.3389/fmolb.2024.1498946
Investigating the crosstalk between ABCC4 and ABCC5 in 3T3-L1 adipocyte differentiation
Abstract
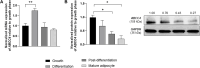
Introduction: The plasma membrane-bound protein, multi-drug resistance-associated protein 4 (MRP4/ABCC4), has gained attention for its pivotal role in facilitating the efflux of a wide range of endogenous and xenobiotic molecules. Its significance in adipogenesis and fatty acid metabolism has been brought to light by recent studies. Notably, research on ABCC4 knockout (ABCC4 -/- ) mice has established a link between the absence of ABCC4 and the development of obesity and diabetes. Nevertheless, the specific contribution of ABCC4 within adipose tissue remains largely unexplored.
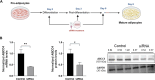
Methods: To address this gap, we conducted a study to elucidate the role of the ABCC4 transporter in mature adipocytes, using siRNA constructs to silence its gene function.
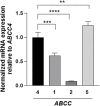
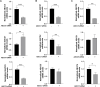
Results: The successful knockdown of ABCC4 significantly altered lipid status and adipogenic gene expression in mature 3T3-L1 adipocytes. Intriguingly, this knockdown also altered the gene expression patterns of other ABCC transporter family members in 3T3-L1 cells. The downregulation of ABCC5 expression was particularly noteworthy, suggesting potential crosstalk between ABCC transporters in mature adipocytes. Additionally, knocking down ABCC5 resulted in significantly higher adipogenic and lipogenic gene expression levels. Oil Red O staining confirmed increased lipid accumulation following the knockdown of ABCC4 and ABCC5. Surprisingly, the simultaneous knockdown of both transporters did not show a cumulative effect on adipogenesis, rather it led to higher levels of intracellular cAMP and extracellular prostaglandin metabolite, both of which are essential signaling molecules in adipogenesis.
Conclusion: These results highlight the complex interplay between ABCC4 and ABCC5 transporters in adipocyte function and suggest their individual contributions toward obesity and related disorders.
Keywords: 3T3-L1 cells; ABCC transporter; adipogenesis; cAMP; lipids; siRNA.
Copyright © 2024 Laddha, Wahane, Bahal and Manautou.
Conflict of interest statement
Author JM is the Boehringer Ingelheim Pharmaceuticals, Inc. Endowed Chair in Mechanistic Toxicology and is supported by an Endowment Fund. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex.Int J Obes (Lond). 2012 May;36(5):725-34. doi: 10.1038/ijo.2011.124. Epub 2011 Jun 28. Int J Obes (Lond). 2012. PMID: 21712809 Free PMC article.
-
Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells.Lipids Health Dis. 2017 Sep 25;16(1):181. doi: 10.1186/s12944-017-0574-7. Lipids Health Dis. 2017. PMID: 28946872 Free PMC article.
-
Torreya nucifera seed oil improves 3T3-L1 adipocyte differentiation.BMC Complement Med Ther. 2021 Oct 7;21(1):255. doi: 10.1186/s12906-021-03429-5. BMC Complement Med Ther. 2021. PMID: 34620154 Free PMC article.
-
Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds.Mol Biol Rep. 2021 Jan;48(1):743-761. doi: 10.1007/s11033-020-06036-8. Epub 2020 Dec 4. Mol Biol Rep. 2021. PMID: 33275195 Review.
-
Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development.Curr Med Chem. 2008;15(20):1981-2039. doi: 10.2174/092986708785132870. Curr Med Chem. 2008. PMID: 18691054 Review.
Cited by
-
Obesity induced transcriptional changes in skeletal muscle across different species.PLoS One. 2025 Jul 14;20(7):e0327988. doi: 10.1371/journal.pone.0327988. eCollection 2025. PLoS One. 2025. PMID: 40658709 Free PMC article.
References
-
- Bertollotto G. M., De Oliveira M. G., Alexandre E. C., Calmasini F. B., Passos G. R., Antunes E., et al. (2018). Inhibition of multidrug resistance proteins by MK 571 enhances bladder, prostate, and urethra relaxation through cAMP or cGMP accumulation. J. Pharmacol. Exp. Ther. 367, 138–146. 10.1124/JPET.118.250076 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

