Booster COVID-19 mRNA vaccination ameliorates impaired B-cell but not T-cell responses in older adults
- PMID: 39717779
- PMCID: PMC11663736
- DOI: 10.3389/fimmu.2024.1455334
Booster COVID-19 mRNA vaccination ameliorates impaired B-cell but not T-cell responses in older adults
Abstract
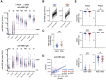
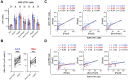
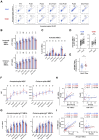
Age-associated differences in the effect of repetitive vaccination, particularly on memory T-cell and B-cell responses, remain unclear. While older adults (aged ≥65 years) exhibited enhanced IgG responses following COVID-19 mRNA booster vaccination, they produced fewer spike-specific circulating follicular helper T cells-1 than younger adults. Similarly, the cytotoxic CD8+ T-cell response remained diminished with reduced PD-1 expression even after booster vaccination compared with that in younger adults, suggesting impaired memory T-cell activation in older adults. In contrast, although B-cell responses in older adults were weaker than those in younger adults in the primary response, the responses were significantly enhanced upon booster vaccination, reaching levels comparable with that observed in younger adults. Therefore, while booster vaccination ameliorates impaired humoral immunity in older adults by efficiently stimulating memory B-cell responses, it may less effectively enhance T-cell-mediated cellular immunity. Our study provides insights for the development of effective therapeutic and vaccine strategies for the most vulnerable older population.
Keywords: COVID-19 article type: original research article; booster vaccination; cTfh; immune aging; immunological memory; mRNA vaccine; memory B cells; memory T cells.
Copyright © 2024 Kometani, Yorimitsu, Jo, Yamaguchi, Kikuchi, Fukahori, Sawada, Tsujimoto, Sunami, Li, Ito, Pretemer, Gao, Hidaka, Yamamoto, Kaku, Nakagama, Kido, Grifoni, Sette, Nagao, Morita, Nakajima, Muto and Hamazaki.
Conflict of interest statement
AlS is a consultant for Darwin Health, EmerVax, Gilead Sciences, Guggenheim Securities, RiverVest Venture Partners, and Arcturus. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

