Polyacrylamide-based hydrogel electrolyte for modulating water activity in aqueous hybrid batteries
- PMID: 39717802
- PMCID: PMC11664367
- DOI: 10.1039/d4ra07551j
Polyacrylamide-based hydrogel electrolyte for modulating water activity in aqueous hybrid batteries
Abstract
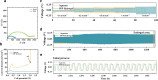
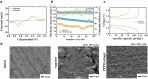
While zinc-ion and hybrid aqueous battery systems have emerged as potential substitutes for expensive lithium-ion batteries, issues like side reactions, limited electrochemical stability, and electrolyte leakage hinder their commercialization. Due to their low cost, high stability, minimal leakage risks, and a wide variety of modification opportunities, hydrogel electrolytes are considered the most promising solution compared to liquid or solid electrolytes. Here, we synthesized a dual-function hydrogel electrolyte based on polyacrylamide and poly(ethylene dioxythiophene):polystyrene (PPP). This electrolyte reduces water content and enhances stability by minimizing side reactions while swelling in a binary ethylene glycol and water solution (EG 10%) further stabilizes the battery system. The developed hydrogel exhibits relatively good ionic conductivity (1.6 × 10-3 S cm-1) and excellent electrochemical stability, surpassing 2.5 V on linear sweep voltammetry tests. The PPP-based system reached a value of 119.2 mA g-1, while the aqueous electrolyte reached only 80.4 mA g-1 specific capacity. The rechargeable PPP hydrogel electrolyte-based hybrid aqueous battery with zinc anode achieved more than 600 cycles. Coulombic efficiency (CE) remained at 99%, indicating good electrochemical reaction stability and reversibility. This study highlights the potential of polyacrylamide-based hydrogel electrolytes with dual functionality as the electrolyte and separator, inspiring further development in hydrogel electrolytes for aqueous battery systems. This study highlights the potential of polyacrylamide-based hydrogel electrolytes with dual functionality as the electrolyte and separator, inspiring further development in hydrogel electrolytes for aqueous battery systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Armand M. Tarascon J.-M. Nature. 2008;451:652–657. - PubMed
-
- Konarov A. Voronina N. Jo J. H. Bakenov Z. Sun Y.-K. Myung S.-T. ACS Energy Lett. 2018;3:2620–2640.
-
- Yan J. Wang J. Liu H. Bakenov Z. Gosselink D. Chen P. J. Power Sources. 2012;216:222–226.
-
- Ayyanusamy P. Tharani S. D. Alphonse R. Minakshi M. Sivasubramanian R. Chem.–Eur. J. 2024;30:e202402325. - PubMed
-
- Ma L. Schroeder M. A. Borodin O. Pollard T. P. Ding M. S. Wang C. Xu K. Nat. Energy. 2020;5:743–749.
LinkOut - more resources
Full Text Sources

