Repurposing of Indomethacin and Naproxen as anticancer agents: progress from 2017 to present
- PMID: 39717807
- PMCID: PMC11664213
- DOI: 10.1039/d4ra07581a
Repurposing of Indomethacin and Naproxen as anticancer agents: progress from 2017 to present
Abstract
Inflammation is strongly linked to cancer and is essential for the growth and development of tumors. Targeting inflammation and the mediators involved in the inflammatory process could therefore provide a suitable method for cancer prevention and therapy. Numerous studies have shown that inflammation can predispose tumors. Non-steroidal anti-inflammatory drugs (NSAIDs) can affect the tumor microenvironment through increasing apoptosis and chemo-sensitivity while decreasing cell migration. Since the development of novel drugs requires a significant amount of money and time and poses a significant challenge for drug discovery, there has been a recent increase in interest in drug repositioning or repurposing. The growing body of research suggests that drug repurposing is essential for the quicker and less expensive development of anticancer therapies. In order to set the course for potential future repositioning of NSAIDs for clinical deployment in the treatment of cancer, the antiproliferative activity of derivatives of Indomethacin and Naproxen as well as their mechanism of action and structural activity relationships (SARs) published in the time frame from 2017 to 2024 are summarized in this review.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
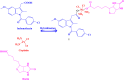
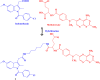
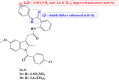
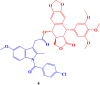
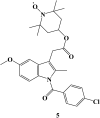
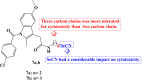
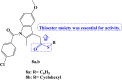
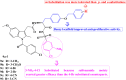
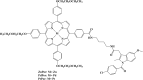
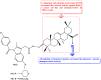
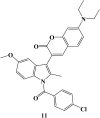


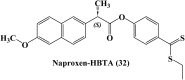
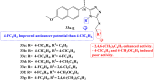
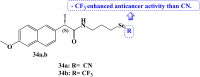
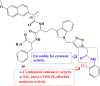
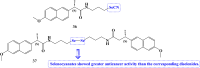
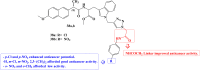
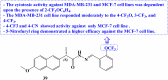
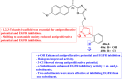

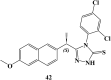
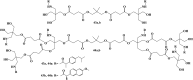
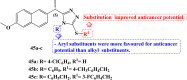
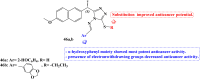
References
-
- Sung H. Ferlay J. Siegel R. L. Laversanne M. Soerjomataram I. Jemal A. Bray F. Ca-Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Whiteman D. C. Wilson L. F. Cancer Epidemiol. 2016;44:203–221. - PubMed
-
- Stanković T. Dinić J. Podolski-Renić A. Musso L. Burić S. S. Dallavalle S. Pešić M. Curr. Med. Chem. 2019;26:6074–6106. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

