Beyond templates: exploring uncharted territory in anisotropic gold nanostructure-oligomer composites synthesis and electrocatalytic performance towards environmental pollutants
- PMID: 39717817
- PMCID: PMC11664328
- DOI: 10.1039/d4ra07744j
Beyond templates: exploring uncharted territory in anisotropic gold nanostructure-oligomer composites synthesis and electrocatalytic performance towards environmental pollutants
Abstract
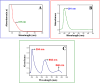
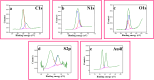
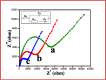
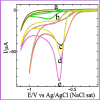
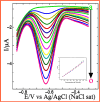
The synthesis of polymer/oligomer-stabilized metal nanostructures (MNS) opens up a wide range of possibilities, from fundamental materials science to practical applications in domains such as medicine, catalysis, sensing, and energy. Because of the versatility of this synthetic approach, it is a dynamic and ever-changing field of study. These polymers/oligomers have precise control over the nucleation and growth kinetics, allowing the production of mono-disperse MNS with well-defined properties. The protective coating provided by polymers or oligomers increased the stability and colloidal dispersity of MNS in these oligomer-MNS composites. As a result, the current research reports the electrocatalytic reduction of nitrobenzene (NB) utilizing oligomeric aminomercaptotriazole (oligo AMTa) and oligo (AMTa-AuNS) modified glassy carbon (GC) electrodes developed via a wet chemical technique. UV-visible spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), high resolution mass spectroscopy (HR-MS), and high-resolution transmission electron microscopy (HR-TEM) approaches were used to confirm the development of oligomer and AuNS. After that, the GC electrode was directly linked to the oligo AMTa and oligo AMTa-AuNS by dipping them in the appropriate solutions. Scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS), and cycle voltammetry (CV) were all employed to confirm the fabrication of oligo AMTa and oligo AMTa-AuNS. Eventually, the electrochemical reduction of NB occurred using the fabricated electrodes. The catalytic activity of oligo AMTa-AuNS has been observed to be more than that of the other modified electrode. As an outcome, the film was employed to determine the sensitivity level of NB, and a limit of detection (LOD) of 2.8 nM was found. The straight-forward method's practical utility was proven by measuring NB in lake sample water.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- George J. M. Antony A. Mathew B. Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchim. Acta. 2018;185:358. - PubMed
-
- Amirjani A. Rahbarimehr E. Recent advances in functionalization of plasmonic nanostructures for optical sensing. Microchim. Acta. 2021;188:57. - PubMed
-
- John A. Benny L. Cherian A. R. Narahari S. Y. Varghese A. Hegde G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: a review. J. Nanostruct. Chem. 2021;11:1.
-
- Sajanlal P. R. Sreeprasad T. S. Nair A. S. Pradeep T. Wires, plates, flowers, needles, and core–shells: diverse nanostructures of gold using polyaniline templates. Langmuir. 2008;24:4607. - PubMed
-
- Kyomuhimbo H. D. Feleni U. Catalytic and energy storage applications of metal/polyaniline nanocomposites: a critical review. J. Electron. Mater. 2022;51:5568.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

