Somatic piRNA and PIWI-mediated post-transcriptional gene regulation in stem cells and disease
- PMID: 39717847
- PMCID: PMC11663942
- DOI: 10.3389/fcell.2024.1495035
Somatic piRNA and PIWI-mediated post-transcriptional gene regulation in stem cells and disease
Abstract
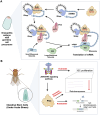
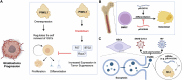
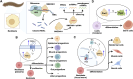
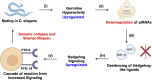
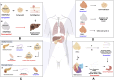
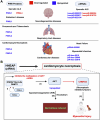
PIWI-interacting RNAs (piRNAs) are small non-coding RNAs that bind to the PIWI subclass of the Argonaute protein family and are essential for maintaining germline integrity. Initially discovered in Drosophila, PIWI proteins safeguard piRNAs, forming ribonucleoprotein (RNP) complexes, crucial for regulating gene expression and genome stability, by suppressing transposable elements (TEs). Recent insights revealed that piRNAs and PIWI proteins, known for their roles in germline maintenance, significantly influence mRNA stability, translation and retrotransposon silencing in both stem cells and bodily tissues. In the current review, we explore the multifaceted roles of piRNAs and PIWI proteins in numerous biological contexts, emphasizing their involvement in stem cell maintenance, differentiation, and the development of human diseases. Additionally, we discussed the up-and-coming animal models, beyond the classical fruit fly and earthworm systems, for studying piRNA-PIWIs in self-renewal and cell differentiation. Further, our review offers new insights and discusses the emerging roles of piRNA-dependent and independent functions of PIWI proteins in the soma, especially the mRNA regulation at the post-transcriptional level, governing stem cell characteristics, tumor development, and cardiovascular and neurodegenerative diseases.
Keywords: PIWI; disease; gene regulation; mRNA; piRNA; retrotransposons; stem cells.
Copyright © 2024 Patel, Jiang and Kakumani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

