Influence of the glycocalyx on the size and mechanical properties of plasma membrane-derived vesicles
- PMID: 39717887
- PMCID: PMC11667464
- DOI: 10.1039/d4sm01317d
Influence of the glycocalyx on the size and mechanical properties of plasma membrane-derived vesicles
Abstract
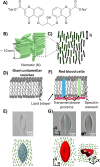
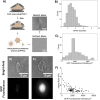
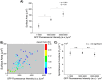
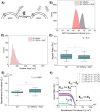
Recent studies have reported that the overexpression of MUC1 glycoproteins on cell surfaces changes the morphology of cell plasma membranes and increases the blebbing of vesicles from them, supporting the hypothesis that entropic forces exerted by MUC1 change the spontaneous curvature of cell membranes. However, how MUC1 is incorporated into and influences the size and biophysical properties of plasma-membrane-blebbed vesicles is not understood. Here we report single-vesicle-level characterization of giant plasma membrane vesicles (GPMVs) derived from cells overexpressing MUC1, revealing a 40× variation in MUC1 density between GPMVs from a single preparation and a strong correlation between GPMV size and MUC1 density. By dispersing GPMVs in aqueous liquid crystals (LCs), we show that the elasticity of the LC can be used to strain individual GPMVs into spindle-like shapes, consistent with the straining of fluid-like membranes. To quantify the influence of MUC1 on membrane mechanical properties, we analyze the shapes of strained GPMVs within a theoretical framework that integrates the effects of MUC1 density and GPMV size on strain. We measure the spontaneous curvature of GPMV membranes to be 2-10 μm-1 and weakly influenced by the 40× variation in MUC1 density, a conclusion we validate by performing independent experiments in which MUC1 is enzymatically removed from GPMVs. Overall, our study advances the understanding of heterogeneity in size and MUC1 density in GPMVs, and establishes single-vesicle-level methods for characterization of mechanical properties within a heterogeneous population of GPMVs. Furthermore, our measurements highlight differences between membrane properties of GPMVs and their parent cells.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Growth Conditions and Cell Cycle Phase Modulate Phase Transition Temperatures in RBL-2H3 Derived Plasma Membrane Vesicles.PLoS One. 2015 Sep 14;10(9):e0137741. doi: 10.1371/journal.pone.0137741. eCollection 2015. PLoS One. 2015. PMID: 26368288 Free PMC article.
-
Cell Line and Media Composition Influence the Production of Giant Plasma Membrane Vesicles.ACS Biomater Sci Eng. 2024 Mar 11;10(3):1880-1891. doi: 10.1021/acsbiomaterials.3c01596. Epub 2024 Feb 19. ACS Biomater Sci Eng. 2024. PMID: 38374716 Free PMC article.
-
Development of Cell-Derived Plasma Membrane Vesicles as a Nanoparticle Encapsulation and Delivery System.bioRxiv [Preprint]. 2023 Aug 7:2023.08.06.552132. doi: 10.1101/2023.08.06.552132. bioRxiv. 2023. PMID: 37609185 Free PMC article. Preprint.
-
Giant plasma membrane vesicles: models for understanding membrane organization.Curr Top Membr. 2015;75:25-57. doi: 10.1016/bs.ctm.2015.03.009. Epub 2015 Apr 17. Curr Top Membr. 2015. PMID: 26015280 Review.
-
Glycocalyx Curving the Membrane: Forces Emerging from the Cell Exterior.Annu Rev Cell Dev Biol. 2021 Oct 6;37:257-283. doi: 10.1146/annurev-cellbio-120219-054401. Annu Rev Cell Dev Biol. 2021. PMID: 34613816 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

