Long-term effects of neoadjuvant chemotherapy in variant histology locally advanced colon cancer: a propensity score-matched analysis
- PMID: 39717890
- PMCID: PMC11702939
- DOI: 10.1080/15384047.2024.2441511
Long-term effects of neoadjuvant chemotherapy in variant histology locally advanced colon cancer: a propensity score-matched analysis
Abstract
Purpose: Neoadjuvant chemotherapy (NAC) has proven valuable in treating locally advanced colon cancer (LACC) and is included as a treatment option for patients with clinical T4b colon cancer by the National Comprehensive Cancer Network. However, the long-term survival benefit of NAC in LACC remains debated, due to a lack of conclusive clinical trial results identifying the patients who would benefit most from NAC. This study aimed to assess the efficacy of NAC in patients with LACC based on histological subtype.
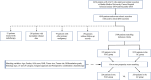
Patients and methods: This retrospective study analyzed 3,709 patients with LACC who underwent curative resection at Harbin Medical University Cancer Hospital between 2014 and 2018. Patients were grouped into two groups: neoadjuvant chemotherapy (NAC) and adjuvant chemotherapy (AC) groups. Propensity score matching (PSM) was used to adjust for confounders, and survival outcomes of the two groups across different histological subtypes were evaluated using Kaplan-Meier (K-M) curves and log-rank tests.
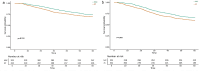
Results: Patients with non-mucinous adenocarcinoma (NMAC) treated with NAC had a significantly improved 5-year OS rate (76.3% vs. 69.2%, p = .039) and DFS rate (67.2% vs. 60.1%, p = .041) compared with patients treated with AC. However, there was no significant difference in OS and DFS between the two treatment groups among patients with mucinous adenocarcinoma (MAC) and signet ring cell carcinoma (SRCC).
Conclusion: In patients with LACC, the prognostic value of NAC varied by histology. NMAC may serve as a predictor of improved long-term survival benefit from NAC in these patients.
Keywords: Colectomy; colon cancer; neoadjuvant chemotherapy; survival.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
