The satiety hormone cholecystokinin gates reproduction in fish by controlling gonadotropin secretion
- PMID: 39717899
- PMCID: PMC11668526
- DOI: 10.7554/eLife.96344
The satiety hormone cholecystokinin gates reproduction in fish by controlling gonadotropin secretion
Abstract
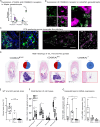
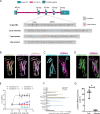
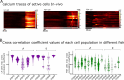
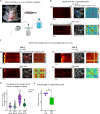
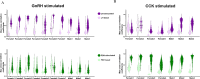
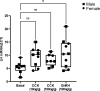
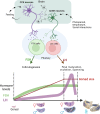
Life histories of oviparous species dictate high metabolic investment in the process of gonadal development leading to ovulation. In vertebrates, these two distinct processes are controlled by the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), respectively. While it was suggested that a common secretagogue, gonadotropin-releasing hormone (GnRH), oversees both functions, the generation of loss-of-function fish challenged this view. Here, we reveal that the satiety hormone cholecystokinin (CCK) is the primary regulator of this axis in zebrafish. We found that FSH cells express a CCK receptor, and our findings demonstrate that mutating this receptor results in a severe hindrance to ovarian development. Additionally, it causes a complete shutdown of both gonadotropins secretion. Using in-vivo and ex-vivo calcium imaging of gonadotrophs, we show that GnRH predominantly activates LH cells, whereas FSH cells respond to CCK stimulation, designating CCK as the bona fide FSH secretagogue. These findings indicate that the control of gametogenesis in fish was placed under different neural circuits, that are gated by CCK.
Keywords: CCK; fsh; neuroscience; pituitary; reproduction; zebrafish.
© 2024, Hollander-Cohen et al.
Conflict of interest statement
LH, OC, MS, TA, PF, OR, PM, MG, BL No competing interests declared
Figures




Update of
- doi: 10.1101/2023.06.18.545454
- doi: 10.7554/eLife.96344.1
- doi: 10.7554/eLife.96344.2
References
-
- Anderson JL, Asche F, Garlock T, Chu J. Aquaculture: Its Role in the Future of Food. World Agricultural Resources and Food Security. Emerald Publishing Limited; 2017. - DOI
-
- Aroua S, Maugars G, Jeng SR, Chang CF, Weltzien FA, Rousseau K, Dufour S. Pituitary gonadotropins FSH and LH are oppositely regulated by the activin/follistatin system in a basal teleost, the eel. General and Comparative Endocrinology. 2012;175:82–91. doi: 10.1016/j.ygcen.2011.10.002. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

