Weekly dihydroartemisinin-piperaquine versus monthly sulfadoxine-pyrimethamine for malaria chemoprevention in children with sickle cell anaemia in Uganda and Malawi (CHEMCHA): a randomised, double-blind, placebo-controlled trial
- PMID: 39718172
- PMCID: PMC12095115
- DOI: 10.1016/S1473-3099(24)00737-0
Weekly dihydroartemisinin-piperaquine versus monthly sulfadoxine-pyrimethamine for malaria chemoprevention in children with sickle cell anaemia in Uganda and Malawi (CHEMCHA): a randomised, double-blind, placebo-controlled trial
Abstract
Background: In many sub-Saharan African countries, it is recommended that children with sickle cell anaemia receive malaria chemoprevention with monthly sulfadoxine-pyrimethamine or daily proguanil as the standard of care. However, the efficacy of these interventions is compromised by high-grade antifolate resistance of Plasmodium falciparum and poor adherence. We aimed to compare the efficacy of weekly dihydroartemisinin-piperaquine and monthly sulfadoxine-pyrimethamine for the prevention of clinical malaria in children with sickle cell anaemia in areas with high-grade sulfadoxine-pyrimethamine resistance of P falciparum in Uganda and Malawi.
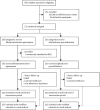
Methods: We did an individually randomised, parallel group, double-blind, placebo-controlled trial at two hospitals in Uganda and two hospitals in Malawi. Children (aged 6 months to 15 years) with sickle cell anaemia with a bodyweight of at least 5kg were randomly assigned (1:1) by computer-generated block randomisation, stratified by site and weight category, to receive either weekly dihydroartemisinin-piperaquine (approximately 2·5 mg per kg bodyweight dihydroartemisinin and 20 mg per kg bodyweight per day piperaquine) or monthly sulfadoxine-pyrimethamine (approximately 25 mg per kg bodyweight sulfadoxine and 1·25 mg per kg bodyweight). Placebos matching the alternative treatment were used in each treatment group to maintain masking of the different dosing schedules from the participants and caregivers, study staff, investigators, and data analysts. All children younger than 5 years received penicillin twice daily as standard of care. The primary endpoint was the incidence of clinical malaria, defined as a history of fever in the preceding 48 h or documented axillary temperature of 37·5°C or higher plus the detection of P falciparum parasites on microscopy (any parasite density). Secondary efficacy outcomes were any malaria parasitaemia (on either microscopy or malaria rapid diagnostic test), all-cause unscheduled clinic visits, all-cause and malaria-specific hospitalisation, sickle cell anaemia-related events (including vaso-occlusive crises, acute chest syndrome, stroke), need for blood transfusion, and death. All primary and secondary outcomes were assessed in the modified intention-to-treat population, which included all participants who were randomly assigned for whom endpoint data were available. Safety was assessed in in all children who received at least one dose of the study drug. Complete case analysis was conducted using negative-binomial regression. This study was registered with Clinicaltrials.gov, NCT04844099.
Findings: Between April 17, 2021, and May 30, 2022, 725 participants were randomly assigned; of whom 724 were included in the primary analysis (367 participants in the dihydroartemisinin-piperaquine group and 357 participants in the sulfadoxine-pyrimethamine group). The median follow-up time was 14·7 months (IQR 11·2-18·2). The incidence of clinical malaria was 8·8 cases per 100 person-years in the dihydroartemisinin-piperaquine group and 43.7 events per 100 person-years in the sulfadoxine-pyrimethamine group (incidence rate ratio [IRR] 0·20 [95% CI 0·14-0·30], p<0·0001). The incidence of hospitalisation with any malaria was lower in the dihydroartemisinin-piperaquine group than the sulfadoxine-pyrimethamine group (10·4 vs 37·0 events per 100 person-years; IRR 0·29 [0·20-0·42], p<0·0001) and the number of blood transfusions was also lower in the dihydroartemisinin-piperaquine group than the sulfadoxine-pyrimethamine group (52·1 vs 72·5 events per 100 person-years; IRR 0·70 [0·54-0·90], p=0·006). The incidence of all-cause unscheduled clinic visits and all-cause hospitalisations were similar between the two groups, however, participants in the dihydroartemisinin-piperaquine group had more clinic visits unrelated to malaria (IRR 1·12 [1·00-1·24], p=0·042) and more hospitalisations with lower respiratory tract events (16·5 vs 8·5 events per 100 person-years; IRR 1·99 [1·25-3·16], p=0·0036) than participants in the sulfadoxine-pyrimethamine group. The number of serious adverse events in the dihydroartemisinin-piperaquine group was similar to that in the sulfadoxine-pyrimethamine group (vaso-occlusive crisis [154 of 367 participants dihydroartemisinin-piperaquine group vs 132 of 357 participants in the sulfadoxine-pyrimethamine group] and suspected sepsis [115 participants vs 92 participants]), with the exception of acute chest syndrome or pneumonia (51 participants vs 32 participants). The number of deaths were similar between groups (six [2%] of 367 participants in the dihydroartemisinin-piperaquine group and eight (2%) of 357 participants in the sulfadoxine-pyrimethamine group).
Interpretation: Malaria chemoprophylaxis with weekly dihydroartemisinin-piperaquine in children with sickle cell anaemia is safe and considerably more efficacious than monthly sulfadoxine-pyrimethamine. However, monthly sulfadoxine-pyrimethamine was associated with fewer episodes of non-malaria-related illnesses, especially in children 5 years or older not receiving penicillin prophylaxis, which might reflect its antimicrobial effects. In areas with high P falciparum antifolate resistance, dihydroartemisinin-piperaquine should be considered as an alternative to sulfadoxine-pyrimethamine for malaria chemoprevention in children younger than 5 years with sickle cell anaemia receiving penicillin-V prophylaxis. However, there is need for further studies in children older than 5 years.
Funding: Research Council of Norway and UK Medical Research Council.
Translations: For the Chichewa, Acholi, Lusoga and Luganda translations of the abstract see Supplementary Materials section.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The Mahidol-Oxford Tropical Medicine Research laboratory, which tested our pharmacokinetic samples, previously did similar paid work for Fosun Pharma (Shanghai, China). RI and JT received travel expenses to attend meetings from Fosun Pharma. All other authors declare no competing interests.
Figures

Similar articles
-
Chemoprevention for malaria with monthly intermittent preventive treatment with dihydroartemisinin-piperaquine in pregnant women living with HIV on daily co-trimoxazole in Kenya and Malawi: a randomised, double-blind, placebo-controlled trial.Lancet. 2024 Jan 27;403(10424):365-378. doi: 10.1016/S0140-6736(23)02631-4. Epub 2024 Jan 12. Lancet. 2024. PMID: 38224710 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effectiveness of sulfadoxine-pyrimethamine plus amodiaquine and dihydroartemisinin-piperaquine for seasonal malaria chemoprevention in Uganda: a three-arm, open-label, non-inferiority and superiority, cluster-randomised, controlled trial.Lancet Infect Dis. 2025 Jul;25(7):726-736. doi: 10.1016/S1473-3099(24)00746-1. Epub 2025 Jan 15. Lancet Infect Dis. 2025. PMID: 39826559 Clinical Trial.
-
Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial.Lancet. 2015 Dec 19;386(10012):2507-19. doi: 10.1016/S0140-6736(15)00310-4. Epub 2015 Sep 28. Lancet. 2015. PMID: 26429700 Free PMC article. Clinical Trial.
-
Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women.Cochrane Database Syst Rev. 2024 Sep 26;9(9):CD006689. doi: 10.1002/14651858.CD006689.pub3. Cochrane Database Syst Rev. 2024. PMID: 39324693 Free PMC article.
Cited by
-
"I want to help my body": Acceptability of malaria chemoprevention among children with sickle cell anaemia and their caregivers in Malawi and Uganda.PLOS Glob Public Health. 2025 Jul 9;5(7):e0004056. doi: 10.1371/journal.pgph.0004056. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 40632789 Free PMC article.
-
The economic burden and catastrophic health expenditures among children with sickle cell anaemia on households in malaria-endemic areas: insights from Uganda and Malawi.BMC Public Health. 2025 Jun 4;25(1):2070. doi: 10.1186/s12889-025-23209-x. BMC Public Health. 2025. PMID: 40468209 Free PMC article.
-
Dihydroartemisinin-piperaquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy: a systematic review and individual participant data meta-analysis.EClinicalMedicine. 2025 Apr 29;83:103202. doi: 10.1016/j.eclinm.2025.103202. eCollection 2025 May. EClinicalMedicine. 2025. PMID: 40370584 Free PMC article.
References
-
- Enato IG, Israel-Aina YT. Proguanil as malaria chemoprophylaxis in sickle cell anaemia: the controversies, problems and the future: a narrative of literature. Niger J Paediatr. 2021;48:104–113.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

