Orientational Disorder and Molecular Correlations in Hybrid Organic-Inorganic Perovskites: From Fundamental Insights to Technological Applications
- PMID: 39718191
- PMCID: PMC11783509
- DOI: 10.1021/acsami.4c12762
Orientational Disorder and Molecular Correlations in Hybrid Organic-Inorganic Perovskites: From Fundamental Insights to Technological Applications
Abstract
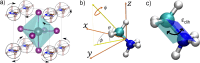
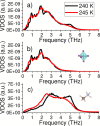
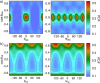
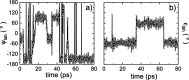
Hybrid organic-inorganic perovskites (HOIP) have emerged in recent years as highly promising semiconducting materials for a wide range of optoelectronic and energy applications. Nevertheless, the rotational dynamics of the organic components and many-molecule interdependencies, which may strongly impact the functional properties of HOIP, are not yet fully understood. In this study, we quantitatively analyze the orientational disorder and molecular correlations in archetypal perovskite CH3NH3PbI3 (MAPI) by performing comprehensive molecular dynamics simulations and entropy calculations. We found that, in addition to the usual vibrational and orientational contributions, rigid molecular rotations around the C-N axis and correlations between neighboring molecules noticeably contribute to the entropy increment associated with the temperature-induced order-disorder phase transition, ΔSt. Molecular conformational changes are equally infrequent in the low-T ordered and high-T disordered phases and have a null effect on ΔSt. Conversely, the couplings between the angular and vibrational degrees of freedom are substantially reinforced in the high-T disordered phase and significantly counteract the phase-transition entropy increase resulting from other factors. Furthermore, the tendency for neighboring molecules to be orientationally ordered is markedly local, consequently inhibiting the formation of extensive polar nanodomains at both low and high temperatures. This theoretical investigation not only advances the fundamental knowledge of HOIP but also establishes physically insightful connections with contemporary technological applications like photovoltaics, solid-state cooling, and energy storage.
Keywords: entropy calculations; hybrid organic–inorganic perovskites; molecular correlations; molecular dynamics simulations; molecular rotational dynamics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
An Unusual Phase Transition Driven by Vibrational Entropy Changes in a Hybrid Organic-Inorganic Perovskite.Angew Chem Int Ed Engl. 2018 Jul 16;57(29):8932-8936. doi: 10.1002/anie.201803176. Epub 2018 Jun 20. Angew Chem Int Ed Engl. 2018. PMID: 29845741
-
Origin of Ferroelectricity in Two Prototypical Hybrid Organic-Inorganic Perovskites.J Am Chem Soc. 2022 Jan 19;144(2):816-823. doi: 10.1021/jacs.1c10188. Epub 2022 Jan 10. J Am Chem Soc. 2022. PMID: 35005965
-
The Effects of the Organic-Inorganic Interactions on the Thermal Transport Properties of CH3NH3PbI3.Nano Lett. 2016 Apr 13;16(4):2749-53. doi: 10.1021/acs.nanolett.6b00457. Epub 2016 Mar 24. Nano Lett. 2016. PMID: 27003760
-
Mix and Match: Organic and Inorganic Ions in the Perovskite Lattice.Adv Mater. 2019 Nov;31(47):e1802697. doi: 10.1002/adma.201802697. Epub 2018 Dec 20. Adv Mater. 2019. PMID: 30570799 Review.
-
Will organic-inorganic hybrid halide lead perovskites be eliminated from optoelectronic applications?Nanoscale Adv. 2019 Jan 16;1(4):1276-1289. doi: 10.1039/c8na00416a. eCollection 2019 Apr 9. Nanoscale Adv. 2019. PMID: 36132615 Free PMC article. Review.
References
-
- Hu L.; Guan X.; Wan T.; Lin C.-H.; Liu S.; Zhu R.; Chen W.; Yao Y.; Huang C.-Y.; Yuan L.; Shahrokhi S.; Chu D.; Cazorla C.; Chen J.; Yang J.; Yi J.; Huang S.; Wu T. Valence-regulated metal doping of mixed-halide perovskites to modulate phase segregation and solar cell performance. ACS Energy Lett. 2022, 7, 4150.10.1021/acsenergylett.2c02040. - DOI
-
- Han C.; Wang Y.; Yuan J.; Sun J.; Zhang X.; Cazorla C.; Wu X.; Wu Z.; Shi J.; Guo J.; Huang H.; Hu L.; Liu X.; Woo H. Y.; Yuan J.; Ma W. Tailoring Phase Alignment and Interfaces via Polyelectrolyte Anchoring Enables Large-Area 2D Perovskite Solar Cells. Angew. Chem., Int. Ed. 2022, 61, e20220511110.1002/anie.202205111. - DOI - PubMed
-
- Guo J.; Sun J.; Hu L.; Fang S.; Ling X.; Zhang X.; Wang Y.; Huang H.; Han C.; Zhou Z.; Cazorla C.; Yang Y.; Chu D.; Wu T.; Yuan J.; et al. Indigo: A Natural Molecular Passivator for Efficient Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2200537.10.1002/aenm.202202288. - DOI
-
- Wang Y.; Duan C.; Zhang X.; Sun J.; Ling X.; Shi J.; Hu L.; Zhou Z.; Wu X.; Han W.; Liu X.; Cazorla C.; Chu D.; Huang S.; Wu T.; Yuan J.; Ma W. Electroluminiscent solar cells based on CsPbI3 perovskites quantum dots. Adv. Funct. Mater. 2022, 32, 2108615.10.1002/adfm.202108615. - DOI
LinkOut - more resources
Full Text Sources

