A unified deep-learning framework for enhanced patient-specific quality assurance of intensity-modulated radiation therapy plans
- PMID: 39718209
- PMCID: PMC11880640
- DOI: 10.1002/mp.17601
A unified deep-learning framework for enhanced patient-specific quality assurance of intensity-modulated radiation therapy plans
Abstract
Background: Modern radiation therapy techniques, such as intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT), use complex fluence modulation strategies to achieve optimal patient dose distribution. Ensuring their accuracy necessitates rigorous patient-specific quality assurance (PSQA), traditionally done through pretreatment measurements with detector arrays. While effective, these methods are labor-intensive and time-consuming. Independent calculation-based methods leveraging advanced dose algorithms provide a reduced workload but cannot account for machine performance during delivery.
Purpose: This study introduces a novel unified deep-learning (DL) framework to enhance PSQA. The framework can combine the strengths of measurement- and calculation-based approaches.
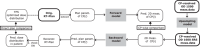
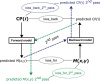
Methods: A comprehensive artificial training dataset, comprising 400,000 samples, was generated based on a rigorous mathematical model that describes the physical processes of radiation transport and interaction within both the medium and detector. This artificial data was used to pretrain the DL models, which were subsequently fine-tuned with a measured dataset of 400 IMRT segments to capture the machine-specific characteristics. Additional measurements of five IMRT plans were used as the unseen test dataset. Within the unified framework, a forward prediction model uses plan parameters to predict the measured dose distributions, while the backward prediction model reconstructs these parameters from actual measurements. The former enables a detailed control point (CP)-wise analysis. At the same time, the latter facilitates the reconstruction of treatment plans from the measurements and, subsequently, dose recalculation in the treatment planning system (TPS), as well as an independent second check software (VERIQA). This method has been tested with an OD 1600 SRS and an OD 1500 detector array with distinct spatial resolution and detector arrangement in combination with a dedicated upsampling model for the latter.
Results: The final models could deliver highly accurate predictions of the measurements in the forward direction and the actual delivered plan parameters in the backward direction. In the forward direction, the test plans reached median gamma passing rates better than 94% for the OD 1600 SRS measurements. The upsampled OD 1500 measurements show similar performance with similar median gamma passing rates but a slightly higher variability. The 3D gamma passing rates from the comparisons between the original and reconstructed dose distributions in patients lie between 95.4% and 98.2% for the OD 1600 SRS and 94.7% and 98.5% for the interpolated OD 1500 measurements. The dose volume histograms (DVH) of the original and the reconstructed plans, recalculated in both the TPS and VERIQA, were evaluated for the organs at risk and targets based on clinical protocols and showed no clinically relevant deviations.
Conclusions: The flexibility of the implemented model architecture allows its adaptability to other delivery techniques and measurement modalities. Its utilization also reduces the requirements of the measurement devices. The proposed unified framework could play a decisive role in automating QA workflow, especially in the context of real-time adaptive radiation therapy (ART).
Keywords: 2D arrays; artificial data; deep learning; patient‐specific quality assurance.
© 2024 The Author(s). Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Efficient dose-volume histogram-based pretreatment patient-specific quality assurance methodology with combined deep learning and machine learning models for volumetric modulated arc radiotherapy.Med Phys. 2022 Dec;49(12):7779-7790. doi: 10.1002/mp.16010. Epub 2022 Oct 17. Med Phys. 2022. PMID: 36190117
-
A method to reconstruct and apply 3D primary fluence for treatment delivery verification.J Appl Clin Med Phys. 2017 Jan;18(1):128-138. doi: 10.1002/acm2.12017. Epub 2016 Dec 8. J Appl Clin Med Phys. 2017. PMID: 28291913 Free PMC article.
-
Applications of machine and deep learning to patient-specific IMRT/VMAT quality assurance.J Appl Clin Med Phys. 2021 Sep;22(9):20-36. doi: 10.1002/acm2.13375. Epub 2021 Aug 3. J Appl Clin Med Phys. 2021. PMID: 34343412 Free PMC article. Review.
-
DVH analysis using a transmission detector and model-based dose verification system as a comprehensive pretreatment QA tool for VMAT plans: Clinical experience and results.J Appl Clin Med Phys. 2019 Nov;20(11):80-87. doi: 10.1002/acm2.12743. Epub 2019 Oct 11. J Appl Clin Med Phys. 2019. PMID: 31605456 Free PMC article.
-
Deep Learning for Radiotherapy Outcome Prediction Using Dose Data - A Review.Clin Oncol (R Coll Radiol). 2022 Feb;34(2):e87-e96. doi: 10.1016/j.clon.2021.12.002. Epub 2021 Dec 16. Clin Oncol (R Coll Radiol). 2022. PMID: 34924256 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

