TAG-1 Regulates NRP1 in Schwann Cells and Participates in Regulating Nerve Regeneration in Rats with Sciatic Nerve Crush Injury
- PMID: 39718654
- PMCID: PMC11668832
- DOI: 10.1007/s11064-024-04310-w
TAG-1 Regulates NRP1 in Schwann Cells and Participates in Regulating Nerve Regeneration in Rats with Sciatic Nerve Crush Injury
Abstract
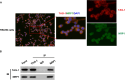
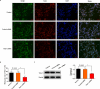
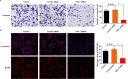
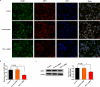
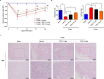
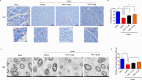
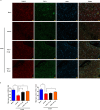
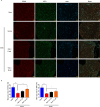
Schwann cells (SCs) are necessary for peripheral nerve regeneration due to their plasticity and trophic supply after sciatic nerve injury (SNI). However, the multiple adaptations of SCs are still poorly understood. This study explored the effects of transient axonal glycoprotein type-1 (TAG-1) on cell migration and neuropilin1 (NRP1) expression in SCs and examined the impact of TAG-1 on nerve regeneration in rats with SNI. The expression of TAG-1 and NRP1 in SCs, RSC96 cells, was measured using co-immunoprecipitation and immunofluorescence. TAG-1 silence in RSC96 cells was established by TAG-1 siRNA transfection. The effects of TAG-1 silence on cell migration and NRP1 expression were measured in cells. Male adult Wistar rats suffered sciatic nerve crush injury and were treated with exogenous TAG-1 protein. The sciatic function was observed every week. The histological changes of sciatic nerves and expressions of S100β, NRP1, GAP43, and NCAM in the nerves were observed after injury 28 days. The TAG-1 and NRP1 were expressed in the RSC96 cells, and there was an interaction between TAG-1 and NRP1. TAG-1 silence suppressed the cell migration and NRP1 expression in cells. In rats with SNI, the TAG-1 treatment improved the sciatic function and promoted nerve regeneration by increasing the expressions of S100β, NRP1, GAP-43, and NCAM in the nerves. This study showed that TAG-1 regulated cell migration and NRP1 expression in SCs, and TAG-1 treatment might be a strategy for nerve regeneration after the SNI.
Keywords: Co-immunoprecipitation; Schwann cells; Sciatic function; Sciatic nerve injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no conflicts of interest to declare. Ethical Approval: All experiment processes were agreed upon with the Ethics Committee of Yantai Yuhuangding Hospital (Approval No. 2024 − 472). All experimental procedures were performed following the ARRIVE guidelines for the animal experiments.
Figures
Similar articles
-
Semaphorin 3E promote Schwann cell proliferation and migration.Exp Cell Res. 2022 Mar 15;412(2):113019. doi: 10.1016/j.yexcr.2022.113019. Epub 2022 Jan 24. Exp Cell Res. 2022. PMID: 35085549
-
Activation of PAR1 contributes to ferroptosis of Schwann cells and inhibits regeneration of myelin sheath after sciatic nerve crush injury in rats via Hippo-YAP/ACSL4 pathway.Exp Neurol. 2025 Feb;384:115053. doi: 10.1016/j.expneurol.2024.115053. Epub 2024 Nov 13. Exp Neurol. 2025. PMID: 39542339
-
KLF7-transfected Schwann cell graft transplantation promotes sciatic nerve regeneration.Neuroscience. 2017 Jan 6;340:319-332. doi: 10.1016/j.neuroscience.2016.10.069. Epub 2016 Nov 5. Neuroscience. 2017. PMID: 27826105
-
α6 and β1 Integrin Heterodimer Mediates Schwann Cell Interactions with Axons and Facilitates Axonal Regeneration after Peripheral Nerve Injury.Neuroscience. 2018 Feb 10;371:49-59. doi: 10.1016/j.neuroscience.2017.11.046. Epub 2017 Dec 6. Neuroscience. 2018. PMID: 29223350
-
Betacellulin regulates peripheral nerve regeneration by affecting Schwann cell migration and axon elongation.Mol Med. 2021 Mar 25;27(1):27. doi: 10.1186/s10020-021-00292-5. Mol Med. 2021. PMID: 33794764 Free PMC article.
Cited by
-
Mitochondrial DNA leakage: underlying mechanisms and therapeutic implications in neurological disorders.J Neuroinflammation. 2025 Feb 7;22(1):34. doi: 10.1186/s12974-025-03363-0. J Neuroinflammation. 2025. PMID: 39920753 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

