Preferential superficial cortical layer activation during seizure propagation
- PMID: 39718688
- PMCID: PMC11908662
- DOI: 10.1111/epi.18239
Preferential superficial cortical layer activation during seizure propagation
Abstract
Objective: Focal cortical seizures travel long distances from the onset zone, but the long-distance propagation pathways are uncertain. In vitro and in vivo imaging techniques have investigated the local spread of seizures but did not elucidate long-distance spread. Furthermore, classical studies in slices suggested seizure spread locally along deep cortical layers, whereas more recent in vivo imaging studies posit a role for superficial cortical layers in local spread.
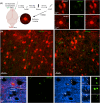
Methods: We imaged seizure-activated neurons using activity reporter mice and measured local field potentials (LFPs) using microelectrode arrays to map cortical seizure propagation in awake mice.
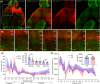
Results: Frontal lobe onset seizures activate more neurons in superficial layers 2-3 than deep layers 5-6 throughout the cortex. LFP recordings demonstrate that seizures spread faster through the superficial than deep layers over long cortical distances of 3.5 mm. We also show that monosynaptically connected long-distance neurons are in the seizure circuit.
Significance: We propose that long-distance cortical seizure spread occurs preferentially via synaptically connected superficial cortical neurons.
Keywords: Layer 2/3; Layer 5/6; deep layers; epilepsy; excitatory synaptic connectivity; local field potentials; seizures; superficial layers.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
J.K. is a consultant for Ovid therapeutics and Marinus Pharmaceuticals. There are no other conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



Similar articles
-
Differential cortical layer engagement during seizure initiation and spread in humans.Nat Commun. 2024 Jun 17;15(1):5153. doi: 10.1038/s41467-024-48746-8. Nat Commun. 2024. PMID: 38886376 Free PMC article.
-
Reliable and Elastic Propagation of Cortical Seizures In Vivo.Cell Rep. 2017 Jun 27;19(13):2681-2693. doi: 10.1016/j.celrep.2017.05.090. Cell Rep. 2017. PMID: 28658617 Free PMC article.
-
Microscale spatiotemporal dynamics during neocortical propagation of human focal seizures.Neuroimage. 2015 Nov 15;122:114-30. doi: 10.1016/j.neuroimage.2015.08.019. Epub 2015 Aug 14. Neuroimage. 2015. PMID: 26279211 Free PMC article.
-
Epileptic seizures recorded with microelectrodes: A persistent multiscale gap between neuronal activity, micro-, and macro-LFP?Rev Neurol (Paris). 2025 Jun;181(6):503-524. doi: 10.1016/j.neurol.2025.01.414. Epub 2025 Apr 30. Rev Neurol (Paris). 2025. PMID: 40312160 Review.
-
Clinical intracranial overview of seizure synchrony and spread.Can J Neurol Sci. 2009 Aug;36 Suppl 2:S55-7. Can J Neurol Sci. 2009. PMID: 19760904 Review.
Cited by
-
Thrombin mediates seizures following cortical injury-induced status epilepticus.Epilepsy Res. 2025 Jul;213:107549. doi: 10.1016/j.eplepsyres.2025.107549. Epub 2025 Apr 1. Epilepsy Res. 2025. PMID: 40188738
-
Seizure Circuit Activity in the Theiler's Murine Encephalomyelitis Virus Model of Infection-induced Epilepsy Using Transient Recombination in Active Populations.bioRxiv [Preprint]. 2025 May 21:2025.05.20.655185. doi: 10.1101/2025.05.20.655185. bioRxiv. 2025. PMID: 40475418 Free PMC article. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

