TRPC1 links calcium signaling to cellular senescence in the protection against posttraumatic osteoarthritis
- PMID: 39718827
- PMCID: PMC11948585
- DOI: 10.1172/jci.insight.182103
TRPC1 links calcium signaling to cellular senescence in the protection against posttraumatic osteoarthritis
Abstract
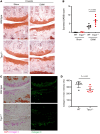
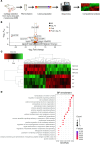
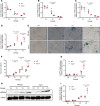
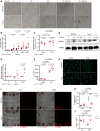
Transient receptor potential channel 1 (TRPC1) is a widely expressed mechanosensitive ion channel located within the endoplasmic reticulum membrane, crucial for refilling depleted internal calcium stores during activation of calcium-dependent signaling pathways. Here, we have demonstrated that TRPC1 activity is protective within cartilage homeostasis in the prevention of cellular senescence-associated cartilage breakdown during mechanical and inflammatory challenge. We revealed that TRPC1 loss is associated with early stages of osteoarthritis (OA) and plays a nonredundant role in calcium signaling in chondrocytes. Trpc1-/- mice subjected to destabilization of the medial meniscus-induced OA developed a more severe OA phenotype than WT controls. During early OA development, Trpc1-/- mice displayed an increased chondrocyte survival rate; however, remaining cells displayed features of senescence including p16INK4a expression and decreased Sox9. RNA-Seq identified differentially expressed genes related to cell number, apoptosis, and extracellular matrix organization. Trpc1-/- chondrocytes exhibited accelerated dedifferentiation, while demonstrating an increased susceptibility to cellular senescence. Targeting the mechanism of TRPC1 activation may be a promising therapeutic strategy in OA prevention.
Keywords: Bone biology; Calcium channels; Cell biology; Cellular senescence; Osteoarthritis.
Conflict of interest statement
Figures



References
-
- OARSI. Osteoarthritis: a serious disease. US Food and Drug Administration. Updated December 1, 2016. Accessed December 19, 2024. https://oarsi.org/sites/oarsi/files/docs/2016/oarsi_white_paper_oa_serio....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

