Mild and Chemoselective Triethylsilane-Mediated Debenzylation for Phosphate Synthesis
- PMID: 39718906
- PMCID: PMC11731398
- DOI: 10.1021/acs.orglett.4c04258
Mild and Chemoselective Triethylsilane-Mediated Debenzylation for Phosphate Synthesis
Abstract
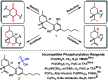
The synthetic utility of tetrabenzyl pyrophosphate for achieving chemoselective phosphorylation of phenols, as well as primary, secondary, and tertiary alcohols, is reported here. Additionally, we introduce a rapid, mild, and chemoselective debenzylation procedure, enabling access to phosphates in the presence of redox sensitive groups. Finally, stoichiometrically controlled monodebenzylation provides a versatile platform for late-stage divergent synthesis of phosphodiester and phosphoramidate chemical libraries.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

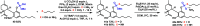
References
-
- Kamerlin S. C. L.; Sharma P. K.; Prasad R. B.; Warshel A. Why Nature Really Chose Phosphate. Q. Rev. Biophys. 2013, 46 (1), 1–132. 10.1017/S0033583512000157. - DOI - PMC - PubMed
- Ardito F.; Giuliani M.; Perrone D.; Troiano G.; Lo Muzio L. The Crucial Role of Protein Phosphorylation in Cell Signaling and its Use as Targeted Therapy. Int. J. Mol. Med. 2017, 40 (2), 271–280. 10.3892/ijmm.2017.3036. - DOI - PMC - PubMed
-
- Rawat P.; Das S.; Shankhdhar D.; Shankhdhar S. C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21 (1), 49–68. 10.1007/s42729-020-00342-7. - DOI
LinkOut - more resources
Full Text Sources

