Linsitinib Decreases Thyrotropin-Induced Thyroid Hormone Synthesis by Inhibiting Crosstalk Between Thyroid-Stimulating Hormone and Insulin-Like Growth Factor 1 Receptors in Human Thyrocytes In Vitro and In Vivo in Mice
- PMID: 39718934
- PMCID: PMC11984798
- DOI: 10.1089/thy.2024.0393
Linsitinib Decreases Thyrotropin-Induced Thyroid Hormone Synthesis by Inhibiting Crosstalk Between Thyroid-Stimulating Hormone and Insulin-Like Growth Factor 1 Receptors in Human Thyrocytes In Vitro and In Vivo in Mice
Abstract
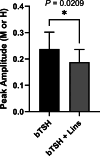
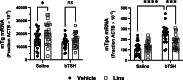
Background: Thyrotropin receptor (TSHR) and insulin-like growth factor 1 receptor (IGF-1R) have been shown to crosstalk in primary cultures of human thyrocytes (hThyros) and Graves' orbital fibroblasts. The phenomenon of TSHR/IGF-1R crosstalk has been largely studied in the pathogenesis of thyroid eye disease (TED) in human orbital fibroblasts. Here, we investigated the effects of inhibiting the IGF-1R-mediated contribution to crosstalk by linsitinib (Lins), a small-molecule IGF-1R kinase inhibitor, on TSH-induced regulation of thyroperoxidase (TPO) and thyroglobulin (TG) mRNAs and proteins in hThyros in vitro, and on TPO and TG mRNAs and free thyroxine (fT4) levels in vivo in mice. Methods: Steady-state levels of mRNAs of TPO and TG in hThyros in vitro and mouse thyroid glands were measured by RT-qPCR. Human TG (hTG) and human TPO (hTPO) proteins in human thyroid cell cultures were measured by Western blot or ELISA. Translation rates of hTG were quantified by stable isotope labeling by amino acids method (SILAC). Thyroidal mouse Tpo (mTpo) and Tg (mTg) mRNAs and fT4 in mice were assessed after Lins administration on 3 consecutive days followed by an intraperitoneal dose of bovine TSH (bTSH) 3 hours prior to drawing blood. Results: In primary cultures of hThyros, Lins inhibited bTSH-induced upregulation of hTPO mRNA by 61.5%, and hTPO protein was inhibited by 42.4%. There was no effect of Lins on hTG mRNA, but Lins inhibited the upregulation of secreted and cell-associated hTG protein by 50.1% and 42.2%, respectively, by inhibiting hTG mRNA translation. mTpo mRNA measured in thyroid glands after treatment with Lins was reduced by 31.5%. There was no effect of Lins on mTg mRNA, however, Lins decreased fT4 levels in mice under basal (endogenous mTSH levels) and bTSH-treated conditions. Conclusions: The IGF-1R antagonist Lins inhibited bTSH-stimulated hTG and hTPO protein expression in primary cultures of hThyros and fT4 levels in mice. We suggest that thyroid function studies be monitored when Lins is administered to humans, for example, if it is used to treat TED.
Keywords: IGF-1R; TSHR; crosstalk; linsitinib; thyroid hormone synthetic genes.
Figures
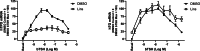



Similar articles
-
Thyrotropin and Insulin-Like Growth Factor 1 Receptor Crosstalk Upregulates Sodium-Iodide Symporter Expression in Primary Cultures of Human Thyrocytes.Thyroid. 2016 Dec;26(12):1794-1803. doi: 10.1089/thy.2016.0323. Epub 2016 Oct 18. Thyroid. 2016. PMID: 27638195 Free PMC article.
-
Thyrotropin, but Not Thyroid-Stimulating Antibodies, Induces Biphasic Regulation of Gene Expression in Human Thyrocytes.Thyroid. 2020 Feb;30(2):270-276. doi: 10.1089/thy.2019.0418. Epub 2020 Jan 28. Thyroid. 2020. PMID: 31805824 Free PMC article.
-
Thyrotropin regulation of differentiated gene transcription in adult human thyrocytes in primary culture.Mol Cell Endocrinol. 2020 Dec 1;518:111032. doi: 10.1016/j.mce.2020.111032. Epub 2020 Sep 14. Mol Cell Endocrinol. 2020. PMID: 32941925 Free PMC article.
-
Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy.Best Pract Res Clin Endocrinol Metab. 2012 Jun;26(3):291-302. doi: 10.1016/j.beem.2011.10.002. Best Pract Res Clin Endocrinol Metab. 2012. PMID: 22632366 Free PMC article. Review.
-
TSH/IGF1 receptor crosstalk: Mechanism and clinical implications.Pharmacol Ther. 2020 May;209:107502. doi: 10.1016/j.pharmthera.2020.107502. Epub 2020 Feb 13. Pharmacol Ther. 2020. PMID: 32061922 Free PMC article. Review.
Cited by
-
Redefining Treatment Paradigms in Thyroid Eye Disease: Current and Future Therapeutic Strategies.J Clin Med. 2025 Aug 6;14(15):5528. doi: 10.3390/jcm14155528. J Clin Med. 2025. PMID: 40807149 Free PMC article. Review.
References
-
- Santisteban P, Kohn LD, Di Lauro R. Thyroglobulin gene expression is regulated by insulin and insulin-like growth factor I, as well as thyrotropin, in FRTL-5 thyroid cells. J Biol Chem 1987;262(9):4048–4052. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
