Biosimilars versus the originator of follitropin alfa for ovarian stimulation in ART: a systematic review and meta-analysis
- PMID: 39719046
- PMCID: PMC11788201
- DOI: 10.1093/humrep/deae274
Biosimilars versus the originator of follitropin alfa for ovarian stimulation in ART: a systematic review and meta-analysis
Abstract
Study question: Is the probability of pregnancy different between women using biosimilars versus the originator of follitropin alfa for ovarian stimulation in ART?
Summary answer: Meta-analysis of eight randomized clinical trials (RCTs) suggests that live birth, clinical, and ongoing pregnancy rates are significantly lower with biosimilars of follitropin alfa compared to the originator.
What is known already: All biosimilars of follitropin alfa have received regulatory approval by demonstrating non-inferiority in the number of retrieved oocytes compared to the originator. Nevertheless, the most clinically relevant outcome in ART for both clinicians and patients is live birth. A meta-analysis published in 2021 suggested that biosimilars of follitropin alfa are associated with lower live birth rates compared to the originator. Since then, more relevant RCTs have been published, and thus an updated critical synthesis of the available evidence is urgently warranted.
Study design, size, duration: A systematic review and meta-analysis were performed to compare biosimilars versus the originator of follitropin alfa in women undergoing ovarian stimulation for ART. A literature search was conducted until January 2024 in MEDLINE, Embase, Cochrane CENTRAL, Scopus, Web of Science, WHO, Clinicaltrials.gov, and others to identify eligible RCTs. The primary outcome was live birth. Secondary outcomes included clinical and ongoing pregnancy, duration of gonadotrophin administration and total FSH dose, number of oocytes retrieved, and ovarian hyperstimulation syndrome (OHSS).
Participants/materials, setting, methods: Data were extracted independently by two reviewers. Quality was assessed using the RoB-2 Tool by Cochrane, and a sensitivity analysis was performed by excluding studies having high risk of bias. Meta-analysis was performed using the random or fixed effects model depending on the presence or not of significant (>50%) statistical heterogeneity (I2). Results were combined using the intention-to-treat principle and are reported as risk ratio (RR) or weighted-mean-difference (WMD) with 95% CIs.
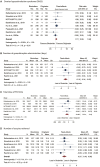
Main results and the role of chance: Eight RCTs (n = 2987) (published between 2015 and 2023) were identified, assessing seven biosimilar products of follitropin alfa. The number of patients included in the eligible studies ranged from 100 to 1100. Three of the RCTs were deemed to be at high risk of bias. The duration of gonadotrophin administration was shorter in the biosimilars group (WMD: -0.19 days, 95% CI: -0.34 to -0.05; I2 = 0%, 5 studies, n = 2081), while no difference was observed in the total dose of FSH (WMD: -34.69 IUs, 95% CI: -74.54 to 5.16; I2 = 15.53%, 5 studies, n = 2081). No difference was observed in the number of oocytes retrieved (WMD: 0.27, 95% CI: -0.43 to 0.96; I2 = 10.7%, 6 studies, n = 1527) and OHSS rates (RR: 1.17, 95% CI: 0.90-1.52; I2 = 0%, 8 studies, n = 2986) between the two groups. A significantly lower live birth rate was observed using the biosimilars of follitropin alfa compared to the originator in women undergoing ovarian stimulation for ART (RR: 0.83, 95% CI: 0.72-0.96; I2 = 0%, 6 studies, n = 2335; moderate certainty of evidence). Similarly, clinical pregnancy (RR: 0.82, 95% CI: 0.73-0.92; I2 = 0%, 7 studies, n = 2876; low certainty of evidence) and ongoing pregnancy rates (RR: 0.81, 95% CI: 0.70-0.94; I2 = 0%, 7 studies, n = 1886; low certainty of evidence) were lower in the biosimilars group. These results were not materially altered in the sensitivity analyses performed where studies deemed at high risk of bias were excluded.
Limitations, reasons for caution: This meta-analysis included RCTs evaluating seven different biosimilars of follitropin alfa; however, pooled data appeared to be homogeneous. No data were available comparing biosimilars of follitropin alfa with the originator regarding cumulative live birth rate per aspiration or the probability of live birth in frozen thawed cycles. The population examined in the eligible RCTs includes mainly normal responders and no RCTs were identified focusing on poor or high responders.
Wider implications of the findings: Clinicians should be informed that although biosimilars of follitropin alfa produce similar number of oocytes with the originator, pregnancy rates after a fresh transfer are likely to be lower. Future research should focus on optimizing the production and use of biosimilars of follitropin alfa, so that they lead to pregnancy rates comparable to the originator.
Study funding/competing interest(s): No external funding was used for this study. K.I.K. and A.S. have no competing interest to disclose. E.M.K. reports personal fees and non-financial support from Merck, Ferring, IBSA, and Vianex. B.W.M. has been supported by an investigator grant from NHMRC, has received consulting fees from Organon, Merck, and Norgine, research support and non-financial support from Merck KGaA, Darmstadt, Germany. B.W.M. also reports having stocks from OBsEva. C.A.V. reports grants, personal fees, and non-financial support from Merck KGaA, Darmstadt, Germany, personal fees, and non-financial support from Merck, Sharpe and Dohme, personal fees and non-financial support from Organon, grants and non-financial support from Ferring, personal fees from IBSA, and personal fees and non-financial support from Gedeon Richter and Vianex.
Registration number: Protocol for the systematic review registered in The International Prospective Register of Systematic Reviews (PROSPERO; CRD42024498237).
Keywords: ICSI; IVF; biosimilars; follitropin-alfa; live birth rate; r-hFSH.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
K.I.K. and A.S. have no competing interest to disclose. E.M.K. reports personal fees and non-financial support from Merck, Ferring, IBSA, and Vianex. B.W.M. has been supported by an investigator grant from NHMRC, has received consulting fees from Organon, Merck, and Norgine, research support and non-financial support from Merck KGaA, Darmstadt, Germany. B.W.M. also reports having stocks from OBsEva. C.A.V. reports grants, personal fees, and non-financial support from Merck KGaA, Darmstadt, Germany, personal fees and non-financial support from Merck, Sharpe and Dohme, personal fees and non-financial support from Organon, grants and non-financial support from Ferring, personal fees from IBSA, personal fees and non-financial support from Gedeon-Richter and Vianex.
Figures


Comment in
-
Follitropin alfa biosimilars: unfounded doubts on the road to reproductive care.Hum Reprod. 2025 Sep 1;40(9):1789-1790. doi: 10.1093/humrep/deaf143. Hum Reprod. 2025. PMID: 40713789 Free PMC article. No abstract available.
-
Methodological concerns and clinical relevance: a critical appraisal of the meta-analysis on biosimilars versus originator follitropin alfa in ART.Hum Reprod. 2025 Sep 1;40(9):1791-1792. doi: 10.1093/humrep/deaf144. Hum Reprod. 2025. PMID: 40713809 No abstract available.
-
Reply: Biosimilars versus the originator of follitropin alfa in ART: eyes wide shut?Hum Reprod. 2025 Sep 1;40(9):1793-1796. doi: 10.1093/humrep/deaf145. Hum Reprod. 2025. PMID: 40713868 No abstract available.
References
-
- Barakhoeva Z, Vovk L, Fetisova Y, Marilova N, Ovchinnikova M, Tischenko M, Scherbatyuk Y, Kolotovkina A, Miskun A, Kasyanova G et al. A multicenter, randomized, phase III study comparing the efficacy and safety of follitropin alpha biosimilar and the original follitropin alpha. Eur J Obstet Gynecol Reprod Biol 2019;241:6–12. - PubMed
-
- Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 2010;25:2092–2100. - PubMed
-
- Braakhekke M, Kamphuis EI, Dancet EA, Mol F, van der Veen F, Mol BW. Ongoing pregnancy qualifies best as the primary outcome measure of choice in trials in reproductive medicine: an opinion paper. Fertil Steril 2014;101:1203–1204. - PubMed
-
- Braam SC, de Bruin JP, Buisman E, Brandes M, Nelen W, Smeenk JMJ, van der Steeg JW, Mol BWJ, Hamilton C. Treatment strategies and cumulative live birth rates in WHO-II ovulation disorders. Eur J Obstet Gynecol Reprod Biol 2018;225:84–89. - PubMed
-
- Chua SJ, Mol BW, Longobardi S, Orvieto R, Venetis CA, Lispi M, Storr A, D’Hooghe T. Biosimilar recombinant follitropin alfa preparations versus the reference product (Gonal-F) in couples undergoing assisted reproductive technology treatment: a systematic review and meta-analysis. Reprod Biol Endocrinol 2021;19:51. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

