Identification of plasma protein biomarkers for endometriosis and the development of statistical models for disease diagnosis
- PMID: 39719050
- PMCID: PMC11788222
- DOI: 10.1093/humrep/deae278
Identification of plasma protein biomarkers for endometriosis and the development of statistical models for disease diagnosis
Abstract
Study question: Can a panel of plasma protein biomarkers be identified to accurately and specifically diagnose endometriosis?
Summary answer: A novel panel of 10 plasma protein biomarkers was identified and validated, demonstrating strong predictive accuracy for the diagnosis of endometriosis.
What is known already: Endometriosis poses intricate medical challenges for affected individuals and their physicians, yet diagnosis currently takes an average of 7 years and normally requires invasive laparoscopy. Consequently, the need for a simple, accurate non-invasive diagnostic tool is paramount.
Study design, size, duration: This study compared 805 participants across two independent clinical populations, with the status of all endometriosis and symptomatic control samples confirmed by laparoscopy. A proteomics workflow was used to identify and validate plasma protein biomarkers for the diagnosis of endometriosis.
Participants/materials, setting, methods: A proteomics discovery experiment identified candidate biomarkers before a targeted mass spectrometry assay was developed and used to compare plasma samples from 464 endometriosis cases, 153 general population controls, and 132 symptomatic controls. Three multivariate models were developed: Model 1 (logistic regression) for endometriosis cases versus general population controls, Model 2 (logistic regression) for rASRM stage II to IV (mild to severe) endometriosis cases versus symptomatic controls, and Model 3 (random forest) for stage IV (severe) endometriosis cases versus symptomatic controls.
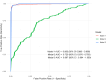
Main results and the role of chance: A panel of 10 protein biomarkers were identified across the three models which added significant value to clinical factors. Model 3 (severe endometriosis vs symptomatic controls) performed the best with an area under the receiver operating characteristic curve (AUC) of 0.997 (95% CI 0.994-1.000). This model could also accurately distinguish symptomatic controls from early-stage endometriosis when applied to the remaining dataset (AUCs ≥0.85 for stage I to III endometriosis). Model 1 also demonstrated strong predictive performance with an AUC of 0.993 (95% CI 0.988-0.998), while Model 2 achieved an AUC of 0.729 (95% CI 0.676-0.783).
Limitations, reasons for caution: The study participants were mostly of European ethnicity and the results may be biased from undiagnosed endometriosis in controls. Further analysis is required to enable the generalizability of the findings to other populations and settings.
Wider implications of the findings: In combination, these plasma protein biomarkers and resulting diagnostic models represent a potential new tool for the non-invasive diagnosis of endometriosis.
Study funding/competing interest(s): Subject recruitment at The Royal Women's Hospital, Melbourne, was supported in part by funding from the Australian National Health and Medical Research Council (NHMRC) project grants GNT1105321 and GNT1026033 and Australian Medical Research Future Fund grant no. MRF1199715 (P.A.W.R., S.H.-C., and M.H.). Proteomics International has filed patent WO 2021/184060 A1 that relates to endometriosis biomarkers described in this manuscript; S.B., R.L., and T.C. declare an interest in this patent. J.I., S.B., C.L., D.I., H.L., K.P., M.D., M.M., M.R., P.T., R.L., and T.C. are shareholders in Proteomics International. Otherwise, the authors have no conflicts of interest.
Trial registration number: N/A.
Keywords: diagnosis; endometriosis; plasma protein biomarkers; proteomics; statistical models.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
Proteomics International has filed patent WO 2021/184060 A1 that relates to endometriosis biomarkers described in this manuscript; S.B., R.L., and T.C. declare an interest in this patent. J.I., S.B., C.L., D.I., H.L., K.P., M.D., M.M., M.R., P.T., R.L., and T.C. are shareholders in Proteomics International. Otherwise, the authors have no conflicts of interest.
Figures


References
-
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. - PubMed
-
- Barberic M, Pavicic Baldani D, Rogic D, Kralik S. Serum concentrations of neuropilin-1 in women with endometriosis. Scand J Clin Lab Invest 2020;80:271–276. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

