Cluster randomised controlled trial of double-dose azithromycin mass drug administration, facial cleanliness and fly control measures for trachoma control in Oromia, Ethiopia: the stronger SAFE trial protocol
- PMID: 39719287
- PMCID: PMC11751794
- DOI: 10.1136/bmjopen-2024-084478
Cluster randomised controlled trial of double-dose azithromycin mass drug administration, facial cleanliness and fly control measures for trachoma control in Oromia, Ethiopia: the stronger SAFE trial protocol
Abstract
Introduction: Trachoma is caused by the bacterium Chlamydia trachomatis (Ct). The WHO recommends the SAFE strategy for trachoma elimination: Surgery for trichiasis, Antibiotics, Facial cleanliness and Environmental improvement. Multiple rounds of SAFE implementation have proven insufficient to eliminate trachoma in Ethiopia, where over 50% of the global trachoma burden remains. More effective antibiotic treatment schedules and transmission-suppressing approaches are needed. The aim of stronger SAFE is to evaluate the impact of a novel package of interventions to strengthen the A, F and E of SAFE on the prevalence of ocular Ct and trachoma in Oromia, Ethiopia.
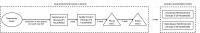
Methods and analysis: 68 clusters were randomised in a 1:1:1:1 ratio to one of (1) standard A/standard F&E (standard SAFE), (2) standard A/enhanced F&E, (3) enhanced A/standard F&E or (4) enhanced A/enhanced F&E (stronger SAFE). Enhanced A includes two height-based doses of oral azithromycin (equivalent to 20 mg/kg) given as single doses 2 weeks apart, as mass drug administration, annually. Enhanced F&E includes fly control measures (permethrin-treated headwear and odour-baited traps) and face-washing hygiene behaviour change implemented at household level in selected communities. The interventions will be implemented and reinforced over 3 years.The primary outcome is the prevalence of ocular Ct by quantitative PCR in children aged 1-9 years at 36 months. A key secondary outcome is the prevalence of active (inflammatory) trachoma in the same children, assessed by validated trachoma graders and conjunctival photography. Laboratory technicians and photo-graders are masked to treatment allocation. Other important secondary analyses include process evaluations, assessment of behaviour change, fly indicators, adherence and coverage of interventions and a cost analysis.
Ethics and dissemination: Study protocols have been approved by the National Research Ethics Review Committee of the Ethiopian Ministry of Science and Higher Education and the London School of Hygiene & Tropical Medicine Ethics Committee. An independent data safety and monitoring board oversees the trial. Results will be disseminated through peer-reviewed publications, presentations and reports.
Trial registration number: ISRCTN40760473.
Keywords: Clinical Trial; Entomology; Epidemiology; Mass Drug Administration; OPHTHALMOLOGY; PUBLIC HEALTH.
© World Health Organization 2024. Licensee BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Water, sanitation, and hygiene for control of trachoma in Ethiopia (WUHA): a two-arm, parallel-group, cluster-randomised trial.Lancet Glob Health. 2022 Jan;10(1):e87-e95. doi: 10.1016/S2214-109X(21)00409-5. Lancet Glob Health. 2022. PMID: 34919861 Free PMC article. Clinical Trial.
-
WASH Upgrades for Health in Amhara (WUHA): study protocol for a cluster-randomised trial in Ethiopia.BMJ Open. 2021 Feb 22;11(2):e039529. doi: 10.1136/bmjopen-2020-039529. BMJ Open. 2021. PMID: 33619183 Free PMC article.
-
Mass azithromycin distribution for hyperendemic trachoma following a cluster-randomized trial: A continuation study of randomly reassigned subclusters (TANA II).PLoS Med. 2018 Aug 14;15(8):e1002633. doi: 10.1371/journal.pmed.1002633. eCollection 2018 Aug. PLoS Med. 2018. PMID: 30106956 Free PMC article. Clinical Trial.
-
Antibiotics for trachoma.Cochrane Database Syst Rev. 2019 Sep 26;9(9):CD001860. doi: 10.1002/14651858.CD001860.pub4. Cochrane Database Syst Rev. 2019. PMID: 31554017 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Geneva: WHO Alliance for the Global Elimination of Trachoma by 2020; 2016. Eliminating trachoma: accelerating towards 2020.
-
- World Health Organization (WHO) WHO alliance for the global elimination of trachoma: progress report on elimination of trachoma, 2022. Weekly epidemiological record, 28. 2023:297–314.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
