Rationale for phosphodiesterase-4 inhibition as a treatment strategy for interstitial lung diseases associated with rheumatic diseases
- PMID: 39719300
- PMCID: PMC11683935
- DOI: 10.1136/rmdopen-2024-004704
Rationale for phosphodiesterase-4 inhibition as a treatment strategy for interstitial lung diseases associated with rheumatic diseases
Abstract
Interstitial lung disease (ILD) associated with rheumatoid arthritis or with connective tissue diseases such as systemic sclerosis can be collectively named systemic autoimmune rheumatic disease-associated ILDs (SARD-ILDs) or rheumatic musculoskeletal disorder-associated ILDs. SARD-ILDs result in substantial morbidity and mortality, and there is a high medical need for effective therapies that target both fibrotic and inflammatory pathways in SARD-ILD. Phosphodiesterase 4 (PDE4) hydrolyses cyclic AMP, which regulates multiple pathways involved in inflammatory processes. PDE4 is overexpressed in peripheral blood monocytes from patients with inflammatory diseases. However, clinical data on pan-PDE4 inhibition in fibrotic conditions are lacking. The PDE4B subtype is highly expressed in the brain, lungs, heart, skeletal muscle and immune cells. As such, inhibition of PDE4B may be a novel approach for fibrosing ILDs such as idiopathic pulmonary fibrosis (IPF) and SARD-ILD. Preclinical data for PDE4B inhibition have provided initial evidence of both anti-inflammatory and antifibrotic activity, with reduced potential for gastrointestinal toxicity compared with pan-PDE4 inhibitors. In a proof-of-concept phase II trial in patients with IPF, nerandomilast (BI 1015550), the only PDE4B inhibitor currently in clinical development, prevented a decline in lung function over 12 weeks compared with placebo. The potential clinical benefit of PDE4B inhibition is now being investigated in the phase III setting, with two trials evaluating nerandomilast in patients with IPF (FIBRONEER-IPF) or with progressive pulmonary fibrosis other than IPF (FIBRONEER-ILD). Here, we review the preclinical and clinical data that provide rationale for PDE4B inhibition as a treatment strategy in patients with SARD-ILD.
Keywords: Autoimmune Diseases; Pulmonary Fibrosis; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MA reports consultancy for Boehringer Ingelheim, Novartis, Pfizer, Roche. OD reports consultancy and/or speaker fees from Abbvie, Arxx, Baecon, Blade, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, MSD, Roche, Roivant, Topadur, UBC. AMHV reports consultancy/speaker and/or grant support from Boehringer Ingelheim, ARXX, Janssen and Roche. MK reports consultancy/speaker and/or grant support from Boehringer Ingelheim, AstraZeneca, Chugai, Kissei, GSK, Mochida, Ono Pharmaceutical, Argenx and Asahai Kasei Pharma. HP reports consultancy/speaker and/or grant support from Boehringer Ingelheim, AstraZeneca, Siemens, Sanofi, Janssen and MSD. ER Volkmann reports consultancy/speaker and/or grant support from Boehringer Ingelheim, CSL Behring, GSK, Roche, Forbus, Prometheus, Savari and Horizon. The authors met criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of this manuscript. Boehringer Ingelheim International GmbH was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
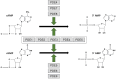
Figures


References
-
- American College of Rheumatology Interstitial lung disease guideline 2023. [29-Nov-2023]. https://rheumatology.org/interstitial-lung-disease-guideline Available. Accessed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
